In some circumstances, dehydrogenation is observed. Dehydrogenation involves the loss of two hydrogen atoms (the reverse of
Question:
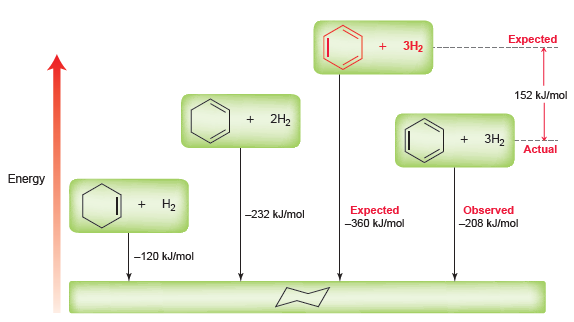
Figure 18.1
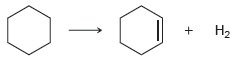
a.
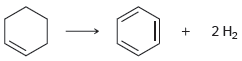
b.
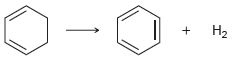
c.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





