Using the data in the following table, predict the sign and magnitude of ÎH° for each of
Question:
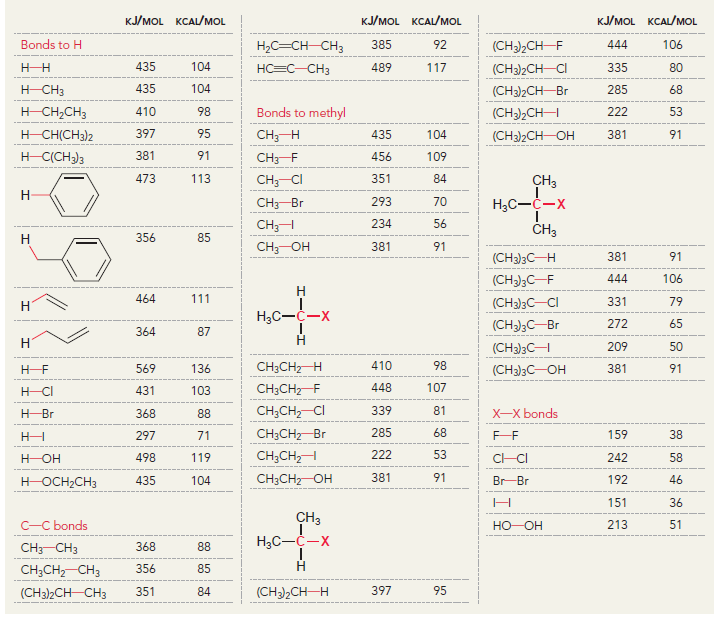
a.
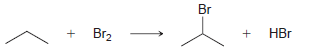
b.

c.
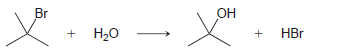
d.

Transcribed Image Text:
KJ/MOL KCAL/MOL к./мOL KCAL/MOL кJ/мOL KCAL/мOL Нас—сн сHз (CH3)2CH F (CH3)2CH CI Bonds to H 385 92 444 106 435 104 НC-С-СНз 489 117 335 80 H CH3 435 104 (CH3)2CH-Br 285 68 н сн-CHз Bonds to methyl 410 98 222 53 (CH3),CHH н СНICH)2 397 95 CHH 435 104 (CH3)2CH-OH 381 91 н ССH)з 381 91 456 109 CH3F CH3 473 113 351 84 CH; CI Н Нас—С—х 293 70 CH3-Br 234 56 CH;H CHз 356 85 Н Cн ОН 381 91 (CH)3C— Н 381 91 (CH)3C-F 444 106 Н 111 464 (CH3)3C-CI 331 79 Н Нас—с—х 272 65 (CHС—Вг 364 87 Н Н (CHд)3C— 209 50 CH3CH2 H 410 98 569 136 (CH)3с—ОН 381 91 448 107 CH3CH2 F H-CI 431 103 CH;CH,-CI 339 81 X-X bonds HBr 368 88 CH3CH2 Br 285 68 159 38 нн 297 71 222 53 CH;CH,H 498 119 C-CI 242 58 H-OH Cн CHz—ОН 381 91 435 192 46 HOCH2CH3 104 Br Br 151 36 CHз C-C bonds 213 51 но-ОН HаС —с —х CH CНз 368 88 Н CH,CH CHЗ 356 85 (CH)2CH H 351 397 95 84 (CH3)2CH CH3 Br Br, НBr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
a Bonds Broken kJmol Bonds Formed kJmol H x CH CH 3 2 397 CH 3 2 CH x Br 285 Br x Br 192 H x Br 368 ...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using the data in the following table, calculate the pI of the following amino acids. (a) Aspartic acid (b) Leucine (c) Lysine (d) Proline THE PK,VALUES FOR TWENTY NATURALLY OCCURRING AMINO ACIDS...
-
Using the data in the following table, calculate the pI of the following amino acids: (a) l-Alanine (b) l-Asparagine (c) l-Histidine (d) l-Glutamic acid THE PK,VALUES FOR TWENTY NATURALLY OCCURRING...
-
Carbon disulfide (CS2) is a toxic, highly flammable substance. The following thermodynamic data are available for CS2(l) and CS2(g) at 298 K: (a) Draw the Lewis structure of the molecule. What do you...
-
Construct the general solution of x ' = Ax involving complex eigenfunctions and then obtain the general real solution. Describe the shapes of typical trajectories. =[ A = 3. -2 1]
-
Provide at least two examples of police corruption as it relates to drugs. Discuss some of the strategies that agencies are implementing to combat corruption in the United States or other countries.
-
A soda vendor at Louisiana State University football games observes that the warmer the temperature at game time the greater the number of sodas that are sold. Based on 32 home games covering five...
-
Consider the delivery time data in Example 3.1. In Section 4.2.5 noted that these observations were collected in four cities, San Diego, Boston, Austin, and Minneapolis. Example 3.1 a. Develop a...
-
The Ajax Coal Company is the only employer in its area. It can hire any number of female workers or male workers it wishes. The supply curve for women is given by Lf = 100wf MEf = Lf =50 and for men...
-
Explain what is meant by corporate governance. In September 2014, Alibaba listed on the New York Stock Exchange in what was, at the time, the largest IPO in history. Explain some of the reasons that...
-
In November 2017, Treasury 4s of 2041 offered a semiannually compounded yield to maturity of 2.6%. Recognizing that coupons are paid semiannually, calculate the bonds price.
-
As mentioned in problem 5.58, some molecules are chiral even though they lack a chirality center. For example, consider the following two compounds shown, and explain the source of chirality in each...
-
For each of the following processes predict the sign of ÎS for the reaction. In other words, will ÎS sys be positive (an increase in entropy) or negative (a decrease in entropy)? a. b. c....
-
The builder of a new movie theater complex is trying to decide how many screens she wants. Below are her estimates of the number of patrons the complex will attract each year, depending on the number...
-
Explain for each event whether it changes the quantity of real GDP demanded or aggregate demand in Japan. The Japanese price level rises. A depreciation of the yen attracts more tourists to Japan. ...
-
Nine longshoremen, all of whom were members of Local 13 of the International Longshoremens and Warehousemens Union, found that they were having difficulty getting jobs. The problem seemed to be that...
-
What are the key provisions of the Civil Rights Act?
-
Bayer took his car to Whitakers auto repair shop for repairs. Whitaker took the car for a test drive, with Bayer seated in the passenger seat. During the test drive, a car operated by another person...
-
What is macroprudential regulation and how does it contrast with micro prudential regulation?
-
Find the length s and area A. Round answers to three decimal places. 4 m
-
Simplify the expression. Assume that all variables are positive. 23VI1 2 V44 8
-
For all practical purposes, the compound cyclohexa-2, 4-dien-1-one exists totally in its enol form. Write the structure of cyclohexa-2, 4-dien-1-one and of its enol form. What special factor accounts...
-
How would you use the acetoacetic ester synthesis to prepare the following?
-
How would you use the acetoacetic ester synthesis to prepare the following?
-
An analysis of the activities needed to produce each product has been conducted. In addition, estimates have been developed. These are in the table below. Match the OH rate with the activity for each...
-
Service business 1. You are required to form a small business (Service business) 2. Explain the name and nature of the business, location, mission and objectives of the company. Assume the business...
-
Cost of utilities Cost per month (In dollars) $20,000 4. The cost of utilities depends on how many wards the hospital needs to use during a particular month. During months with activity under 2,000...

Study smarter with the SolutionInn App


