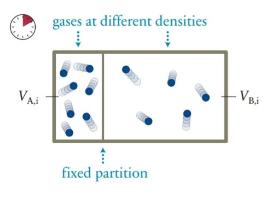
The box in Figure 19.19 contains seven gas particles in compartment (mathrm{A}) and five in compartment (mathrm{B}),
Question:
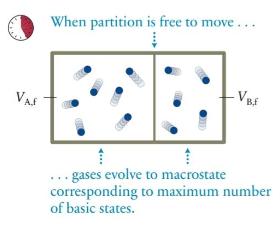
The box in Figure 19.19 contains seven gas particles in compartment \(\mathrm{A}\) and five in compartment \(\mathrm{B}\), and the partition separating the compartments is free to move. Let the volume of the box be \(V\) and the initial ratio of the compartment volumes be \(V_{\mathrm{B}, \mathrm{i}} / V_{\mathrm{A}, \mathrm{i}}=3\). What is the change in the entropy of this gas as it evolves from this macrostate to equilibrium?
Data from Figure 19.19
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





