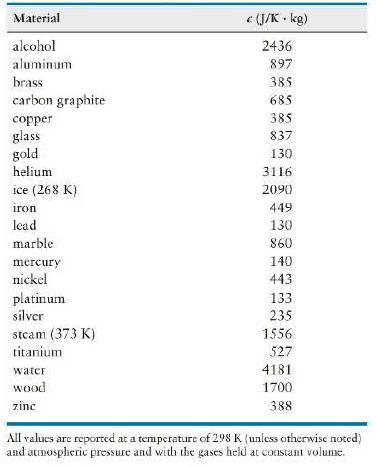
Which material in Table 20. 2 has the greatest specific heat capacity? The smallest? Imagine that objects
Question:
Which material in Table 20. 2 has the greatest specific heat capacity? The smallest? Imagine that objects made of these materials are placed on your hand. The objects have the same mass and are initially at the same temperature. Each object equilibrates thermally with your hand, which is at a higher temperature. Describe any differences in the equilibration process.
Data from Table 20. 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





