A city with a population of 200,000 people operates a 20:0 10 6 gal/day wastewater treatment
Question:
A city with a population of 200,000 people operates a 20:0 × 106 gal/day wastewater treatment plant.
For every million gallons of wastewater treated, 1875 lbm of solids are generated, with 75% of the solids being classified as “volatile” (meaning they are converted to gases during digestion).
Solids generated in the treatment plant are fed to an anaerobic digester in which microorganisms break down biodegradable material in the absence of oxygen. The feed to the digester contains 45,000 mg solids/L at 55°F and has a density approximately that of water. The digester operates at 95°F, converts 50% of the volatile solids to a biogas containing 65 vol% CH4 and 35% CO2 at a rate of 15 SCF (standard cubic feet) gas per lbm of solids converted, and loses approximately 250,000 Btu/h of heat to the surroundings. To supply the heat needed to raise the feed temperature from 55°F to 95°F and to make up for the heat loss, a stream of sludge is pumped from the digester through a heat exchanger in which it comes into thermal contact with a stream of hot water. The heated sludge is returned to the digester. The digester biogas is fed to a furnace in which a fraction of it is burned to heat the water for the heat exchanger from 160°F to 180°F.
A schematic of part of the process is shown below.
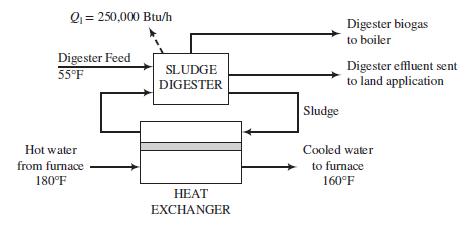
(a) Calculate the rate (SCF/h) at which biogas is produced in the digester and the total heating value (Btu/h) of the gas (= fuel flow rate lower heating value).
(b) Calculate the rate of heat transfer (Btu/h) between the hot water and sludge and the volumetric flow rate (ft3/h) of the water passing through the heat exchanger. Assume the heat of reaction of the anaerobic digestion process is negligible.
(c) If the biogas is burned in a boiler with 80% efficiency (that is, 80% of the heating value of the fuel goes to produce hot water for the heat exchanger), what fraction of the digester gas must be burned to heat the water from 160°F to 180°F? What happens to the other 20% of the heating value?
(d) If there is excess digester gas available after meeting the process–water heating demand, what are its potential uses?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





