Estimate the vapor pressure of acetone (mm Hg) at 50C (a) from data in Perrys Chemical Engineers
Question:
Estimate the vapor pressure of acetone (mm Hg) at 50°C (a) from data in Perry’s Chemical Engineers’ Handbook (Footnote 1) and the Clausius–Clapeyron equation, (b) from the Antoine equation using parameters from Table B.4, and (c) using the AntoineP function in APEx.
Table B.4
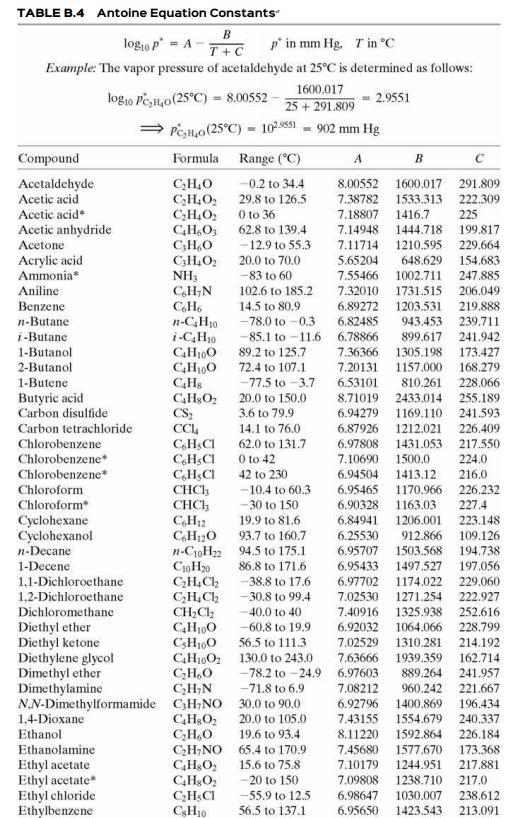
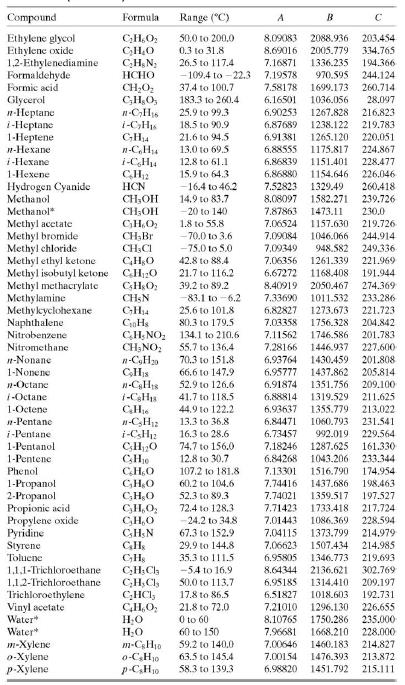
TABLE B.4 Antoine Equation Constants" B log,o p = A p' in mm Hg. Tin °C T+C Example: The vapor pressure of acetaldehyde at 25°C is determined as follows: 1600.017 log10 Pe,H,o(25°C) = 8.00552 25 + 291.809 2.9551 Pen,o(25°C) = 10s51 - 902 mm Hg Compound Formula Range (°C) A B Acctaldehyde Acetic acid C,H,0 C;H,O, C,H,O2 CH,O, C;H,O C3H,O2 NH3 C,H;N -0.2 to 34.4 8.00552 1600.017 291.809 29.8 to 126.5 O to 36 62.8 to 139.4 7.38782 1533.313 222.309 Acetic acid* 7.18807 1416.7 225 Acetic anhydride Acetone 7.14948 1444.718 1210.595 648.629 1002.711 199.817 - 12.9 to 55.3 7.11714 229,664 Acrylic acid Ammonia* 20.0 to 70.0 5.65204 154.683 -83 to 60 7.55466 247.885 Aniline 102.6 to 185.2 7.32010 1731.515 206.049 Benzene 14.5 to 80.9 -78.0 to -0.3 6.89272 1203.531 219.888 n-Butane 6.82485 943.453 n-C,H30 i-C,H10 239.711 i-Butane -85.1 to -11.6 6.78866 89.2 to 125.7 72.4 to 107.1 -77.5 to -3.7 899.617 241.942 1-Butanol 2-Butanol 1-Butene 7.36366 1305.198 173.427 168.279 CH0 CHs 7.20131 1157.000 6.53101 810.261 8.71019 2433.014 1169.110 1212.021 228.066 Butyric acid Carbon disulfide 20.0 to 150.0 3.6 to 79.9 255.189 241.593 CS; CC, C,HSCI C,H;CI C,H;CI CHCH CHCI; C,H12 C,H120 n-C10H2 94.5 to 175.1 CioH20 C;H,Ch 6.94279 Carbon tetrachloride 14.1 to 76.0 6.87926 226.409 Chlorobenzene 62.0 to 131.7 6.97808 1431.053 217.550 Chlorobenzene* 0 to 42 7.10690 1500.0 224.0 Chlorobenzene* 42 to 230 6.94504 1413.12 216.0 Chloroform -10.4 to 60.3 6.95465 1170.966 226.232 Chloroform -30 to 150 6.90328 1163.03 227.4 223.148 Cyclohexane Cyclohexanol 19.9 to 81.6 6.84941 1206.001 93.7 to 160.7 6.25530 912.866 109.126 n-Decane 194.738 6.95707 6.95433 1497.527 1503.568 1-Decene 86.8 to 171.6 197.056 1,1-Dichloroethane 1,2-Dichloroethane Dichloromethane - 38.8 to 17.6 6.97702 1174.022 229.060 -30.8 to 99.4 7.02530 1271.254 222.927 CH;Cl2 C,H100 C;H100 GH1002 130.0 to 243.0 CH,O C;H,N 40.0 to 40 7.40916 1325.938 1064.066 1310.281 252.616 228.799 Diethyl ether Diethyl ketone Diethylene glycol Dimethyl ether Dimethylamine N.N-Dimethylformamide C;H;NO 30.0 to 90.0 -60.8 to 19.9 56.5 to 111.3 6.92032 7.02529 214.192 -78.2 to -24.9 6.97603 -71.8 to 6.9 7.63666 1939.359 889.264 960.242 162.714 241.957 7.08212 221.667 6.92796 1400.869 7.43155 1554.679 196.434 1,4-Dioxane Ethanol C,H&O; CH,O C;H;NO 65.4 to 170.9 20.0 to 105.0 240.337 19.6 to 93.4 8.11220 1592.864 226.184 Ethanolamine 7.45680 1577.670 173.368 Ethyl acetate Ethyl acetate Ethyl chloride Ethylbenzene 15.6 to 75.8 7.10179 1244.951 217.881 -20 to 150 7.09808 1238.710 217.0 C;H;CI CH10 -55.9 to 12.5 6.98647 1030.007 238.612 56.5 to 137.1 6.95650 1423.543 213.091
Step by Step Answer:
Estimation of vapor pressure Vapor pressure is a measure of tendency of material change into the gas...View the full answer



Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
At 300C, the vapor pressure of Hg is 32.97 torr. What mass of Au would have to be dissolved in 5.00 g of Hg to lower its vapor pressure to 25.00 torr?
-
At 300C, the vapor pressure of Hg is 32.97 torr. If 0.775 g of Au were dissolved into 3.77 g of Hg, what would be the vapor pressure of the solution?
-
The Dew Point The vapor pressure of water (see Problem 18.88) decreases as the temperature decreases. If the amount of water vapor in the air is kept constant as the air is cooled, a temperature is...
-
Find the equation for the lower half of the circle x + (y-8) = 7. Put the equation in the form y = g(x), and then enter g(x) into the answer box below. Enter your answer as a symbolic function of x,...
-
Refer to Figure 12.8, which indicates ÎG for each glycolytic reaction under intracellular conditions. Assume that glyceraldehyde-3-phosphate dehy-drogenase was inhibited with iodoacetate, which...
-
If they increase, which of the following items are debited and which are credited: expenses, revenue, assets, liabilities, capital, profits, and losses?
-
You just learned about financing and accounting. Consider how finance and accounting relate to other areas of business. What information would you need to plan for how finance and accounting impacts...
-
Valentine Investigations has the following information for its cash account: Balance, 1/31 ............... $ 7,444 Deposits during February ........... 106,780 Checks written during February ...........
-
Name four characteristics of Force?
-
It should be possible to implement general semaphores using binary semaphores. We can use the operations semWaitB and semSignalB and two binary semaphores, delay and mutex. Consider the following:...
-
The vapor pressure of ethylene glycol at several temperatures is given below: (a) Construct a semilog plot of the vapor-pressure data and determine a linear expression for ln p* as a function of...
-
Estimate the vapor pressure of acetone (mm Hg) at 50C (a) from data in Perrys Chemical Engineers Handbook (Footnote 1) and the ClausiusClapeyron equation, (b) from the Antoine equation using...
-
Briefly describe the nature of the information provided by each of the following financial statements: the income statement, the retained earnings statement, the balance sheet, and the statement of...
-
You have decided to buy a perpetual bond. The bond makes one payment at the end of every year forever and has an interest rate of 5%. If the bond initially costs $2000, what is the payment every year?
-
Discuss in detail the steps that must be carried out to transform the data for the Cochrane-Orcutt method of correcting for autocorrelation.
-
You receive a $11,000 check from your grandparents for graduation. You decide to save it toward a down payment on a house. You invest it earning 12% per year and you think you will need to have...
-
Suppose you currently have $5200 in your savings account, and your bank pays interest at a rate of 0.45% per month. If you make no further deposits or withdrawals, how much will you have in the...
-
What is a direct skip?
-
Multiple-Concept Example 1 uses an approach similar to that needed in this problem, except here the temperature remains constant while a phase change occurs. A sample of liquid water at 100 C and 1...
-
Outline a general process applicable to most control situations. Using this, explain how you would develop a system to control home delivery staff at a local pizza shop.
-
A mixture of sulfuric acid and nitric acid will produce small quantities of the nitronium ion (NO2 + ): Does the nitronium ion have any significant resonance structures? Why or why not? o==o:
-
Consider the structure of ozone: Ozone is formed in the upper atmosphere, where it absorbs short-wavelength UV radiation emitted by the sun, thereby protecting us from harmful radiation. Draw all...
-
Identify the reagents you would use to achieve each of the following transformations: a) Convert tert-butyl bromide into a primary alkyl halide b) Convert 2-bromopropane into 1-bromopropane
-
21. Based on the Gordon Growth Model of stock prices, how would you expect each of the following shocks to affect the overall level of stock prices (up, down, or no change), all else equal? In each...
-
7. (5 Points) The graph plots the value of the firm, VL, aganst the amount of Debt, D. Three lines corresponding to three different stories have been drawn: MM I without taxes, MM I with taxes and...
-
Did you know that 90% of brand interactions are through digital channels? And that most of these digital channels can be found on mobile? make a blog entry (five hundred words minimum) that tells the...

Study smarter with the SolutionInn App


