For protein denaturation, the excess entropy of denaturation is defined as is the transition excess heat capacity.
Question:
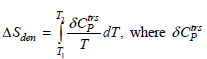
is the
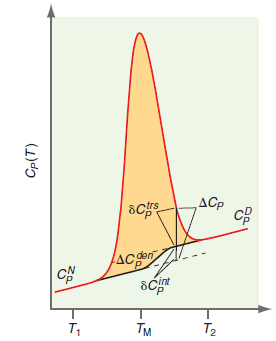
transition excess heat capacity. The way in which δC trs P can be extracted from differential scanning calorimetry (DSC) data is discussed in Section 4.6 and shown in Figure 4.7. The following DSC data are for a protein mutant that denatures between T1 = 288 K and T2 = 318 K. Using the equation for ΔS den given previously calculate the excess entropy of denaturation. In your calculations, use the dashed curve as the heat capacity base line which defines δC trs P as shown in Figure 4.8. Assume the molecular weight of the protein is 14000. grams.
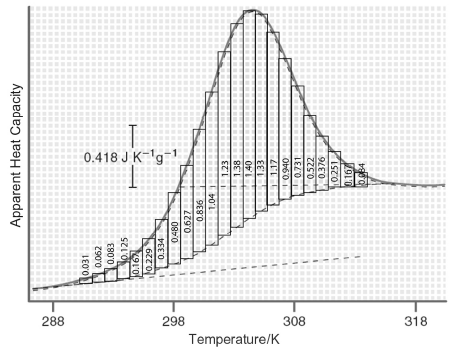
You can perform the integration numerically by counting squares.
Figure 4.7
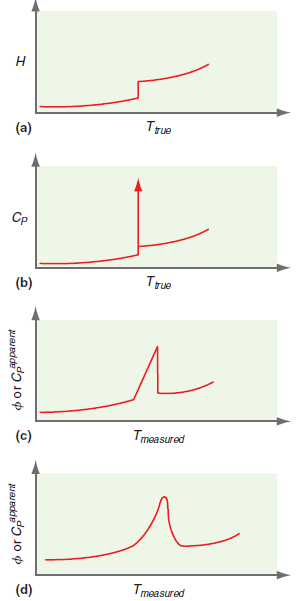
Figure 4.8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





