The standard entropy of Pb(s) at 298.15 K is 64.80 J K -1 mol - 1 .
Question:
The standard entropy of Pb(s) at 298.15 K is 64.80 J K-1mol-1. Assume that the heat capacity of Pb(s) is given by
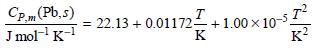
The melting point is 327.4°C and the heat of fusion under these conditions is 4770. J mol-1. Assume that the heat capacity of Pb(l) is given by
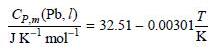
a. Calculate the standard entropy of Pb(l) at 725°C.
b. Calculate ΔH for the transformation Pb(s, 25.0°C) †’ Pb(l, 725°C).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





