Peptide bond hydrolysis is performed by a family of enzymes known as serine proteases. The name is
Question:
a. The cleavage of NBT by chymotrypsin was studied and the following reaction rates were measured as a function of substrate concentration:
![NBT] (mM) 1.00 2.00 4.00 6.00 8.00 Rateg(mM s) 0.062 0.107 0.040 0.082 0.099](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/6/4/3245afbff4459dcf1526464313988.jpg)
Use these data to determine Km and Rate max for chymotrypsin with NBT as the substrate
b. The cleavage of NAT was also studied and the following reaction rates versus substrate concentration were measured:
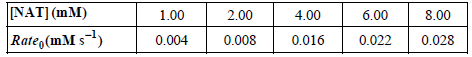
Use these data to determine Km and rate max for chymotrypsin with NAT as the substrate.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





