Pure ethane is burned completely with preheated 20% excess air. The combustion product gas passes through an
Question:
Pure ethane is burned completely with preheated 20% excess air. The combustion product gas passes through an insulated heat exchanger (the preheater) in which it transfers heat to the air that will continue on to the furnace. The following data are recorded for the preheater inlet and outlet streams:
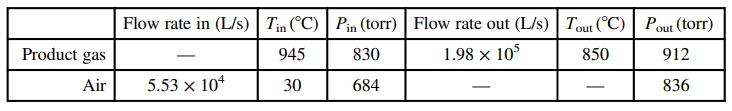
The temperature of the air leaving the preheater is not known because the thermocouple mounted in that pipe has malfunctioned.
(a) Calculate the molar flow rates (mol/s) of the two streams flowing through the preheater and the temperature of the air leaving the preheater.
(b) The preheated air thermocouple is replaced and gives a reading of 161°C. List possible reasons for the discrepancy between what you predicted and what was measured. (Think about all of the assumptions built into your calculations.)
(c) Careful re-measurements are made of the process stream flow rates, temperatures, and pressures, and all of the given values are replicated. What is the most likely reason for the difference between the measured exit air temperature and the temperature predicted in Part (a)? What would you recommend to correct the problem?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





