Question:
Sixty-five liters of liquid ethanol at 70.0°C and 55 L of liquid water at 20.0°C are to be mixed in a wellinsulated flask. The energy balance for this constant pressure process is Q = ΔH.
(a) Neglecting evaporation and the heat of mixing, estimate the final mixture temperature. (As part of the calculation, use data in Table B.2 to estimate a linear formula for the heat capacity of liquid ethanol.)
(b) If the experiment were actually performed and the final mixture temperature were measured, it would almost certainly not equal the value estimated in Part (a). List as many reasons as you can to explain the observation. (There are at least seven of them, most involving approximations made in the estimation.)
able B.2
![TABLE B.2 Heat Capacities Form 1: C,[kJ/(mol-C)] or [kJ/(mol-K)] = a + bT + cr + dT Form 2: C,k/(molC)j or [kJ/(mol-K)j = a + bT + cT2 Example: (C,)acetone(e) = 0.07196 + (20.10 x 10-5)r - (12.78 x 10-8)7? + (34.76 x 10-12)Tr*, where T is in °C. %3D](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/0/6/3/4035ec6712b530b51590063382161.jpg)
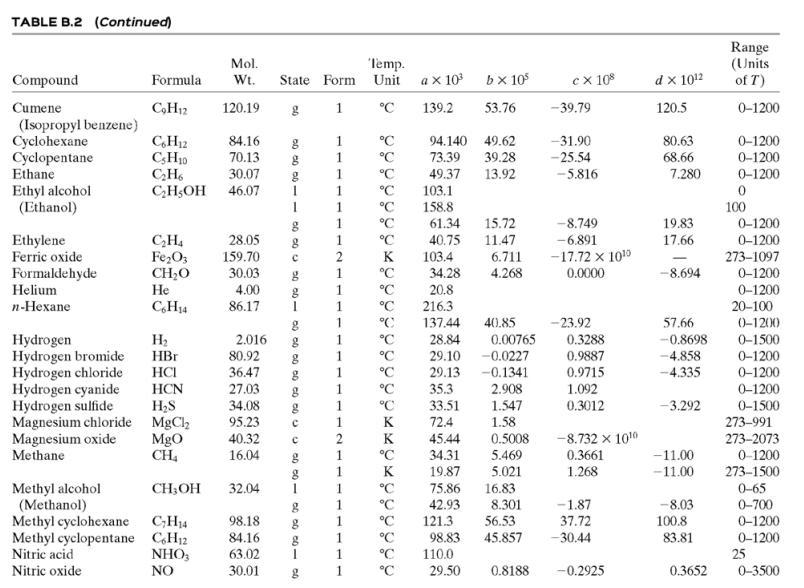
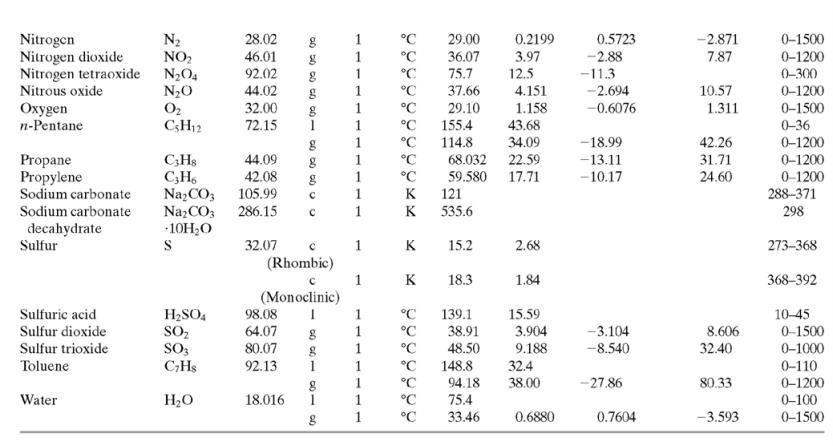
Transcribed Image Text:
TABLE B.2 Heat Capacities Form 1: C,[kJ/(mol-C)] or [kJ/(mol-K)] = a + bT + cr + dT Form 2: C,k/(molC)j or [kJ/(mol-K)j = a + bT + cT2 Example: (C,)acetone(e) = 0.07196 + (20.10 x 10-5)r - (12.78 x 10-8)7? + (34.76 x 10-12)Tr*, where T is in °C. %3D Note: The formulas for gases are strictly applicable at pressures low enough for the ideal-gas equation of state to apply. Range (Units of T) Mol. Temp. State Form Unit a x 10 ex 10 d x 1012 Compound Formula Wt. bx 10 Acetone CH;COCH; 58.08 1 °C 123.0 18.6 -30-60 °C 71.96 34.76 20.10 6.053 0.4147 -12.78 -5.033 0.3191 0-1200 0-1200 0-1500 C;H2 26.04 29.0 °C 42.43 28.94 28.09 18.20 -1.965 1.965 -6.686 Acetylene Air 0.1965 0.4799 273-1800 Ammonia NH, 17.03 1 °C 35.15 2.954 0.4421 0-1200 Ammonium sulfate Benzene (NH4),SO4 132.15 C,H, 215.9 275-328 6-67 126.5 74.06 78.11 1 °C 23.4 32.95 -25.20 77.57 0-1200 CH10 CH10 CHs CaC2 CACO, Ca(OH)2 CaO Isobutane 58.12 89.46 49.87 30.13 27.88 -18.91 0-1200 0-1200 n-Butane 58.12 92.30 -15.47 34.98 Isobutene 56.10 82.88 25.64 -17.27 50.50 0-1200 Calcium carbide Calcium carbonate Calcium hydroxide 64.10 68.62 -8.66 x 1010 -12.87 x 1010 2 K 1.19 298-720 100.09 K 82.34 89.5 4.975 273-1033 74.10 K 276-373 -4.52 x 1010 -4.891 x 1010 -2.887 Calcium oxide 56.08 12.01 K 41.84 2.03 273-1173 Carbon с 11.18 1.095 273-1373 CO2 CO Carbon dioxide 44.01 36.11 4.233 7.464 0-1500 Carbon monoxide 28.01 1. 28.95 0.4110 0.3548 -2.220 0-1500 Carbon tetrachloride CC4 Ch 153.84 93.39 12.98 1.367 0.6117 273-343 0-1200 273-1357 Chlorine 70.91 1 °C 33.60 -1.607 6.473 Соpper Cu 63.54 1. K 22.76 212 21-
![TABLE B.2 Heat Capacities Form 1: C,[kJ/(mol-C)] or [kJ/(mol-K)] = a + bT + cr + dT Form 2: C,k/(molC)j or [kJ/(mol-K)j = a + bT + cT2 Example: (C,)acetone(e) = 0.07196 + (20.10 x 10-5)r - (12.78 x 10-8)7? + (34.76 x 10-12)Tr*, where T is in °C. %3D](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/0/6/3/4035ec6712b530b51590063382161.jpg)








