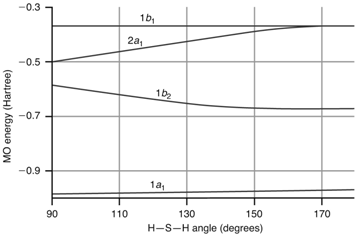
The energy of the occupied valence MOs of H 2 S is shown as a function of
Question:
For comparison, also offer a bond-angle explanation based on hybridization rather than on the MO diagram.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





