The following data show how the standard molar constant-pressure heat capacity of sulfur dioxide varies with temperature.
Question:
The following data show how the standard molar constant-pressure heat capacity of sulfur dioxide varies with temperature. By how much does the standard molar enthalpy of SO2(g) increase when the temperature is raised from 298.15K to 1500K?
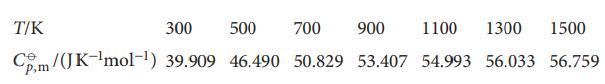
Transcribed Image Text:
T/K 300 500 700 900 1100 1300 1500 Cm/(JK-¹mol-¹) 39.909 46.490 50.829 53.407 54.993 56.033 56.759
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (13 reviews)
Answer and thorough explanation The standard molar enthalpy of a substance is given by H Hf CpT Tf w...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
The following data show how the marginal external benefit and marginal private benefit associated with a soil treatment agent to control Japanese beetles vary with the gallons of the control agent...
-
The following data show the exam grades for two different sections of a statistics class that I teach. The first group of data is from a day section of traditional students. The second group of data...
-
The following data show the daily closing prices (in dollars per share) for IBM for November 3, 2005, through December 1, 2005 (Compustat, February 26, 2006). a. Define the independent variable...
-
As manager of a local pizza parlor, you want to develop a balanced scorecard so you can more effectively monitor the restaurants performance. Required a. Propose at least two goals for each...
-
Using the information from BE14- 24, prepare the journal entry to record the bond issue, assuming that Lee Equipment Company is an IFRS reporter. BE14-24 Lee Equipment Company issued 200 eight- year,...
-
(b) What method did the companies use in adopting the new lease accounting standard, ASC842? Which line items on the financial statements are affected the most by the adoption? Amongthe four...
-
The beam shown is tapered along its width. If a force \(\mathbf{P}\) is applied to its end, determine the strain energy in the beam and compare this result with that of a beam that has a constant...
-
Presented below is information related to Bobby Engram Company. Instructions (a) Compute the ending inventory at retail. (b) Compute a cost-to-retail percentage (round to two decimals) under the...
-
As Grandparents, parents, sisters, brothers, and productive citizens of society, we are always concerned that our children do not hang out with the so-called wrong crowd. Let me tell you of the...
-
Boyd Docker engaged in the following activities in establishing his photography studio, SnapShot!: 1. Opened a bank account in the name of SnapShot! and deposited $8,000 of his own money into this...
-
Describe two calorimetric methods for the determination of enthalpy changes that accompany chemical processes.
-
A sample consisting of 1.00mol Ar is expanded isothermally at 20 C from 10.0dm 3 to 30.0dm 3 (i) Reversibly, (ii) Against a constant external pressure equal to the final pressure of the gas, (iii)...
-
Solve Problem by using any method. 2x 2 = 6x - 3
-
Chandra was the sole shareholder of Pet Emporium, which was originally formed as an S corporation. When Pet Emporium terminated its S election on August 31, 2018, Chandra had a stock basis and an...
-
Cathy, Heathcliff, and Isabelle are equal shareholders in Wuthering Heights (WH), an S corporation. Heathcliff has decided he would like to terminate the S election. In the following alternative...
-
Carmen SanDiego, a U.S. citizen, is employed by General Motors Corporation, a U.S. corporation. On April 1, 2019, GM relocated Carmen to its Brazilian operations for the remainder of 2019. Carmen was...
-
Jane has been operating Mansfield Park as a C corporation and decides she would like to make an S election. What is the earliest the election will become effective under each of these alternative...
-
Janna has a tax basis of $15,000 in her Mimikaki stock (Mimikaki has been an S corporation since inception). In 2019, Janna was allocated $20,000 of ordinary income from Mimikaki. What is the amount...
-
As mentioned in Chapter 3, ammonium nitrate is the most important nitrogen-containing fertilizer in the world. Given only air and water as starting materials and any equipment and catalyst at your...
-
Some people argue that the internal control requirements of the Sarbanes-Oxley Act (SOX) put U.S. companies at a competitive disadvantage to companies outside the United States. Discuss the...
-
Calculate the percentage difference in the fundamental vibration wavenumber of 23 Na 35 Cl and 23 Na 37 Cl on the assumption that their force constants are the same.
-
At low resolution, the strongest absorption band in the infrared absorption spectrum of 12 C 16 O is centred at 2150 cm 1 . Upon closer examination at higher resolution, this band is observed to be...
-
An object of mass 1.0 kg suspended from the end of a rubber band has a vibrational frequency of 2.0 Hz. Calculate the force constant of the rubber band.
-
A positive charge q = +8 nC is at the origin, and a second positive charge q = +12 nC is on the x axis at a = 4 m, Find the net electric field (a) at point P, on the x axis at x=7 m, and (b) at point...
-
8. Hooten Carpentry had the following accounts and account balances after adjusting entries. Assume all accounts have normal balances. Prepare the adjusted trial balance for Hooten Carpentry as of...
-
Explain at least one major difference that exists today between US GAAP and IFRS on the accounting for foreign currency transactions. Be specific.

Study smarter with the SolutionInn App


