The isomerization of cyclopropane over a limited pressure range was examined in Problem 20B.14. If the Lindemann
Question:
The isomerization of cyclopropane over a limited pressure range was examined in Problem 20B.14. If the Lindemann mechanism of unimolecular reactions is to be tested we also need data at low pressures. These have been obtained (H.O. Pritchard et al., Proc. R. Soc. A 217, 563 (1953)):
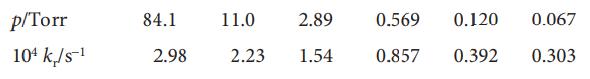
Test the Lindemann–Hinshelwood theory with these data.
Data in Problem 20B.14.
Cyclopropane isomerizes into propene when heated to 500 °C in the gas phase. The extent of conversion for various initial pressures has been followed by gas chromatography by allowing the reaction to proceed for a time with various initial pressures:

where p0 is the initial pressure and p is the final pressure of cyclopropane. What is the order and rate constant for the reaction under these conditions?
Step by Step Answer:

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula





