The partial pressures of Br 2 above a solution containing CCl 4 as the solvent at 25°C
Question:
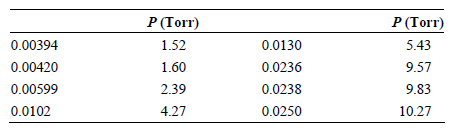
Transcribed Image Text:
P (Torr) 1.52 P (Torr) 5.43 9.57 9.83 0.00394 0.00420 0.0130 0.0236 0.0238 0.0250 1.60 2.39 0.00599 0.0102 4.27 10.27
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
The best fit line in the plot is PBr ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Your task in this problem will be to use a spreadsheet to generate a Txy diagram for a two-component system, using Raoult?s law to express the vapor?liquid equilibrium distribution of each species....
-
A 1.00- L gas sample at 100oC and 600. torr contains 50.0% helium and 50.0% xenon by mass. What are the partial pressures of the individual gases?
-
The partial pressures of an equilibrium mixture of N2O4(g) and NO2(g) are PN2O4 = 0.34 atm and PNO2 = 1.20 atm at a certain temperature. The volume of the container is doubled. Calculate the partial...
-
Crystal Cleaners dry cleans industrial clothing. The following excerpt from its PPE Subledger shows the component details regarding the dry cleaning equipment: Calculate depreciation on the dry...
-
Consider the following modifications to the Good and Rich Candy Company example from class. How would the aggregate planning strategy change in each case provided? Data from class lecture include:...
-
An article 4 in www.networkworld.com about evaluating e-mail filters that are designed to detect spam described a test of Mail Frontiers Anti-Spam Gateway (ASG). In the test, there were 7840 spam...
-
A petrol engine with bore \(150 \mathrm{~mm}\) and stroke \(200 \mathrm{~mm}\) has a clearance volume of 700 litres. If the indicated thermal efficiency is 0.30 , find the relative efficiency. Take...
-
Wellstone Companys Small Motor Division manufactures a number of small motors used in household and office appliances. The Household Division of Wellstone then assembles and packages such items as...
-
What are the challenges associated with conducting hazard analysis in multi-disciplinary environments, where processes involve a mix of mechanical, electrical, and chemical systems? How can...
-
Wardell Company purchased a mini computer on January 1, 2019, at a cost of $48,500. The computer has been depreciated using the straight-line method over an estimated five-year useful life with an...
-
A and B form an ideal solution. At a total pressure of 0.720 bar, y A = 0.510 and x A = 0.420. Using this information, calculate the vapor pressure of pure A and of pure B.
-
The data from Problem P9.20 can be expressed in terms of the molality rather than the mole fraction of Br2 . Use the data from the following table and a graphical method to determine the Henrys law...
-
Find the Jacobian of the transformation. x = uv, y = vw, z = wu
-
You have just deposited $1000 in an unusual bank account that pays interest biannually (once every 2 years). If the 2-year interest rate is 10% (total interest over 2 years is 10%, not 10% per year),...
-
Determine the moment of inertia of a homogeneous circular corona, of surface density \(\sigma=1.25\) \(\mathrm{kg} \mathrm{m}^{-2}\), with inner radius \(r_{1}=0.30 \mathrm{~m}\) and outer radius...
-
Find an \(N\) th-order AR approximation for the ARMA system \[H(z)=\frac{1-1.5 z^{-1}}{1+0.5 z^{-1}}\] and compare the resulting magnitude response to the original ARMA system. Observe that in this...
-
On a thin bar of negligible mass \(1 \mathrm{~m}\) long are arranged 5 bodies each of mass \(0.10 \mathrm{~kg}\) at distances of \(25 \mathrm{~cm}\) from each other starting from one end. Determine...
-
Design a bandpass filter satisfying the specification below using the Hamming, Hann, and Blackman windows: \[\begin{aligned}M & =10 \\\Omega_{\mathrm{c}_{1}} & =1.125 \mathrm{rad} /...
-
1. How many people could you let know that you are searching for a job? List their names and how you know them (e.g., your internship mentor or a former boss). 2. How would you ask them to help you...
-
Why is inventory management important for merchandising and manufacturing firms and what are the main tradeoffs for firms in managing their inventory?
-
Calculate the standard Gibbs energy of the reaction 4 HCl(g) + O2(g) 2 Cl 2 (g) + 2 H 2 O(l) at 298 K, from the standard entropies and enthalpies of formation given in the Data section.
-
Suggest a physical interpretation of the dependence of the Gibbs energy on the temperature.
-
The enthalpy of vaporization of chloroform (CHCl 3 ) is 29.4 kJ mol 1 at its normal boiling point of 334.88 K. Calculate (a) The entropy of vaporization of chloroform at this temperature and (b) The...
-
AA stock price is $45/share. The stock is expected to pay dividends $2.50 in the coming year. After reviewing all information, you concluded: the price one year from now will be $50/share and you...
-
Fung is planning for the correct after tax and after inflation real rate of return to use. If inflation will be 1.3%, the average rate of return on his investments will be 4.9%, and the tax rate on...
-
In a three - level marketing channel, how many entities ( organization / individual ) are connected?

Study smarter with the SolutionInn App


