The temperaturecomposition diagram for the Ca/Si binary system is shown in Fig. 5.7. (a) Identify eutectics, congruent
Question:
The temperature–composition diagram for the Ca/Si binary system is shown in Fig. 5.7.
(a) Identify eutectics, congruent melting compounds, and incongruent melting compounds.
(b) If a 20 per cent by atom composition melt of silicon at 1500 °C is cooled to 1000 °C, what phases (and phase composition) would be at equilibrium? Estimate the relative amounts of each phase.
(c) Describe the equilibrium phases observed when an 80 per cent by atom composition Si melt is cooled to 1030 °C. What phases, and relative amounts, would be at equilibrium at a temperature
(i) Slightly higher than 1030 °C,
(ii) Slightly lower than 1030 °C? Draw a graph of the mole percentages of both Si(s) and CaSi2(s) as a function of mole percentage of melt that is freezing at 1030 °C.
Data in Fig. 5.7.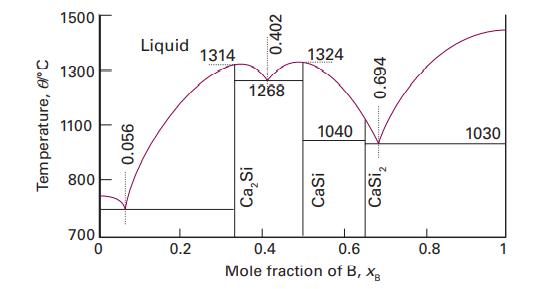
Step by Step Answer:

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula





