The vapor pressures of 1-bromobutane and 1-chlorobutane can be expressed in the form And Assuming ideal solution
Question:
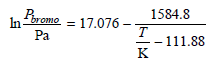
And
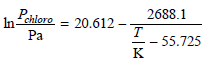
Assuming ideal solution behavior, calculate x bromo and y bromo at 305 K and a total pressure of 9750. Pa. At 305 K, Po bromo = 7113 Pa and Po chloro = 18552 Pa.
Transcribed Image Text:
1584.8 Poromo = 17.076 - In т 111.88 K Pa 2688.1 Pehlore Pa 20.612 – In- т - 55.725 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Ptotal Xbromo Promo 1 Xbromo Pehla c...View the full answer

Answered By

Shivani Gupta
I have done mtech from very reputed college .i am very passionate about teaching.i have more than 2 years of experience in teaching physics and prepared them for competitive exams like IIT-JEE and neet exams. I make physics easy to students as they think physics is difficult for them.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
x[n] is a real-valued, nonnegative, finite-length sequence of length N; i.e., x[n] is real and nonnegative for 0 n N 1 and is zero otherwise. The N-point DFT of x[n] is X[k], and the Fourier...
-
The sawtooth waveform in figure can be expressed in the form of a Fourier series as(a) Determine the Fourier series coefficients ck.(b) Use an N-point subroutine to generates samples of this signal...
-
The Heisenberg uncertainty principle can be expressed in the form where E represents energy and t represents time. Show that the units for this form are the same as the units for the form used in...
-
Alpha Appliance Service had net income for the year of $ 35,000. In addition, the balance sheet reports the following balances: Calculate the return on assets (ROA) for Alpha Appliance Service for...
-
Berry company manufactures two products, (1) Regular (2) Deluxe. The budgeted units to be produced are as follows: 2015 Regular Deluxe January 10,000 15,000 February 6,000 10,000 March 9,000 14,000...
-
Consider a regression explaining the share of Olympic Games medals won by each country in 1988 (in Seoul, South Korea), 1992 (in Barcelona, Spain), and 1996 (in Atlanta, GA, USA) as a function of \(L...
-
Barry Randolph is a global marketing manager for Gen Tech Corporation, a leading manufacturer of computer accessories. The company has market-leading products in several categories, including...
-
For the extraction of Cu 2+ by dithizone in CCl 4 , KL= 1.1 10 4 , K M = 7 10 4 , K a = 3 10 - 5 , = 5 10 22 , and n = 2. (a) Calculate the distribution coefficient for extraction of 0.1 M Cu 2+...
-
Consider the cubic polynomial P3(x) = 1 + x-2x + x, and rewrite it by the following polynomial: 3 93(x) = a; (x 1). j=0 Do so by determining the unknown constants a; for all j = {0, 1, 2, 3}. Use...
-
Lee Appliances knows that weekly demand for high-end microwaves is normally distributed, with a mean of 25 units and a standard deviation of 7 units. (In your model use integers for all demands.) Lee...
-
A volume of 5.50 L of air is bubbled through liquid toluene at 298 K, thus reducing the mass of toluene in the beaker by 2.38 g. Assuming that the air emerging from the beaker is saturated with...
-
Construct a stem-and-leaf plot of the cholesterol changes? Table 2.15: Serum-cholesterol levels (mg/dL) before and after adopting a vegetarian diet *Before after. Subject Before After Difference* 195...
-
Is it possible for a company's profit from operations and its profit to be the same amount? If so, should any company bother to calculate both numbers?
-
Describe the accessing functions for one-dimensional arrays at the implementation level.
-
True or False? O(N*N) is called quadratic time.
-
What characterizes functional decomposition?
-
The specifications for the Sorted List ADT state that the item to be deleted is in the list. 1. Rewrite the specification for DeleteItem so that the list is unchanged if the item to be deleted is not...
-
Explain how an expert understanding of your programming language can reduce the amount of time you spend debugging.
-
What will the code in Figure 4-54 assign to the lblStatus control when the strLevel variable contains the string 3? a. Bronze b. Gold c. Platinum d. Silver If strLevel = "1" OrElse strLevel "2" Then...
-
An item of depreciable machinery was acquired on 1 July 2009 for $120,000 by cash It is expected to have a useful life of 10 years and zero salvage value On 1 July 2012, it was decided to revalue the...
-
Calculate the standard enthalpies of formation of (a) KClO 3 (s) from the enthalpy of formation of KCl, (b) NaHCO 3 (s) from the enthalpies of formation of CO 2 and NaOH together with the following...
-
Calculate the standard enthalpy of solution of AgCl(s) in water from the enthalpies of formation of the solid and the aqueous ions.
-
When 120 mg of naphthalene, C 10 H 8 (s), was burned in a bomb calorimeter the temperature rose by 3.05 K. Calculate the calorimeter constant. By how much will the temperature rise when 10 mg of...
-
Explain organizational change and briefly discuss the three types of change? ( 350 words please)
-
Identify an organization that has experienced change Classify the type of organizational change the organization experienced Describe how the organization overcame the resistance to the change...
-
Carlton Bank has an increase in reserves of $1,000,000. If the reserve ratio is 10%, by what amount may Carlton increase its demand deposits?

Study smarter with the SolutionInn App


