Use the vapor pressures for tetrachloromethane given in the following table to estimate the temperature and pressure
Question:
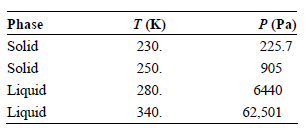
Transcribed Image Text:
P (Pa) T (K) Phase Solid 230. 225.7 Solid 905 250. Liquid 6440 280. Liquid 340. 62,501
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
To estimate H sublimation use the vapor pressures of the solid phase To estimate H vaporizati...View the full answer

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the vapor pressures of tetrachloromethane given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P/Pa T (K) 320. 330....
-
Use the vapor pressures for hexane given in the following table to estimate the temperature and pressure of the triple point and also the enthalpies of fusion, vaporization, and sublimation. Phase T...
-
The temperature dependence of the vapour pressure of solid sulfur dioxide can be approximately represented by the relation log (p/Torr) = 10.5916 - 1871.2/ (T/K) and that of liquid sulfur dioxide by...
-
Locate the centroid of the region bounded by the given curves. about x = 5, y = 2x2, y = 0, x = 5 < UseHorizontal ElementOf Area > x = 5 y = 2x? y = 0
-
Your supervisor in the finance department at Adaptive Solutions Online has asked you to create a worksheet for the flagship product that will project the annual gross margin, total expenses,...
-
When fractional factorial design is useful?
-
Why might an auditor elect to perform only limited or no tests of controls for fixed assets?
-
The adjusted trial balance columns of the worksheet for Pisa Company are as follows. InstructionsComplete theworksheet. PISA COMPANY Worksheet (partial) For the Month Ended April 30, 2012 Adjusted...
-
Marketing Tactics (The 4 p's.) 1. Spells out how marketing strategies will be turned into specific action programs that answer the following questions: What will be done? When will it be done? Who is...
-
A car has a mass of 900 kg. It accelerates from rest at a rate of 1.2 m/s 2 . a. Calculate the time taken to reach a velocity of 30 m/s. b. Calculate the force required to accelerate the car at a...
-
In Section 8.8, it is stated that the maximum height of a water column in which cavitations does not occur is 9.7 m. Show that this is the case at 298 K.
-
Within what range can you restrict the values of P and T if the following information is known about CO 2 ? Use Figure 8.12 to answer this question. a. As the temperature is increased, the solid is...
-
The report One in Three American Households Are Stuck in a Relationship with a Financial Services Provider They Dont Trust (businesswire.com/news /home/20160629005198/en/American-Households...
-
Sunland Records wants to sell enough records to earn a profit of $139200. If the unit selling price is $22, unit variable cost is $10, and total fixed costs are $208800, how many records must Sunland...
-
Sally earns gross wages of $1200 per week. She has standard deductions for social security and medicare. She is single with 1 allowance. She also has 6% of her gross wages put into a retirement...
-
During 2022, Illini, Inc. was involved in pending litigation due to defective products sold during the year. As of December 31, 2022, Illini, Inc. estimated a loss of $1,500,000 was probable. In...
-
Use the definition of the derivative (AKA the four step process) to find the derivative of f(x) = x - 7x+6 ==
-
Warranty expense is expected to be 2% of sales and should be accrued in the period of the sale. The 2022 beginning and ending balances of the company's warranty liability were $24,000 and $28,000,...
-
Evaluate the integral. tan-1 dx x?
-
Copy and complete the statement. 3800 m ? km =
-
Use the derivative theorem to derive the Laplace transform of cos (at) from the Laplace transform of sin (at).
-
An object falling in a vacuum near the surface of the earth experiences a gravitational force in the z direction given by F z = mg, where g is called the acceleration due to gravity and is equal to...
-
Show that the function of Eq. (12.25) satisfies Eq. (12.12). z = b 1 cos(Ït) + b 2 sin(Ït). (12.25) dz (12.12) -kz. dt2
-
Laker Company reported the following January purchases and sales data for its only product. For specific identification, ending inventory consists of 280 units from the January 30 purchase, 5 units...
-
Solve: 4 = log 625
-
Simplify. (-8x2+2x-3)-[(4x2 - 1x + - [(4x - 1x+8) 2(-x + x + 3)] - G

Study smarter with the SolutionInn App


