Use the vapor pressures of tetrachloromethane given in the following table to calculate the enthalpy of vaporization
Question:
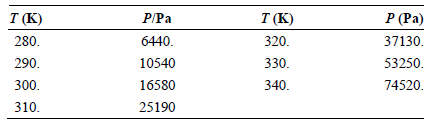
Transcribed Image Text:
P/Pa T (K) 320. 330. P (Pa) 37130. T (K) 280. 6440. 53250. 74520. 290. 10540 300. 16580 340. 25190 310.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
A least squares fit of ln P ver...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the vapor pressures for tetrachloromethane given in the following table to estimate the temperature and pressure of the triple point and also the enthalpies of fusion, vaporization, and...
-
Use the values for nominal GDP and real GDP given in the following table to calculate the inflation rate during 1930: 1929 5103.6 billion 5977.0 billion 1930 Nominal GDP Real GDP $91.2 billion $892.8...
-
Use the vapor pressures of ice given here to calculate the enthalpy of sublimation using a graphical method or a least squares fitting routine. T (K) P (Torr) 200. 0.1676 210. 0.7233 2.732 220. 230....
-
The JoFe Computers and Accessory Company produces two types of laptop computer bags. Version A costs $32, takes 4 hours of labor, and sells for $50. Version B costs $38, takes 6 hours of labor, and...
-
1). Critically analyze current European and United States industry standards or recommendations for any Information Technology (IT) area or subarea (e.g., intrusion detection, data recovery, data...
-
Cancer is caused by genes gone awryyet cancer is not usually an inherited genetic condition. Explain why.
-
Two plates are at temperatures of \(T_{1}\) and \(T_{2}\) and a chemical reaction is producing heat at a constant rate within the system. Derive a model to predict the temperature distribution within...
-
Miranda, Inc., manufactures and sells commercial and residential security equipment. The comparative unclassified balance sheets for December 31, 2011 and 2010 are provided below. Selected missing...
-
IVANHOE COMPANY Trial Balance August 31, 2022 Before Adjustment After Adjustment Cr. Dr. Cash $10,246 Cr. Dr. $10.246 Accounts Receivable 8,272 8.836 Supplies 2,350 470 Prepaid Insurance 3,760 2,350...
-
According to data from the National Highway Traffic Safety Administration, the accident rate as a function of the age of the driver in years x can be approximated by the function (x) = 0.0232x 2 -...
-
20.0 g of water is in a container of 20.0 L at 298.15 K. The vapor pressure of water at this temperature is 23.76 Torr. a. What phases are present? b. At what volume would only the gas phase be...
-
The vapor pressure of ethanol(l) is given by a. Calculate the standard boiling temperature. b. Calculate ÎH vaporization at 298 K and at the standard boiling temperature. 3.6745 x 10 23.58 In...
-
Use the following figure to answer the questions below. a. Is the slope of Line A positive or negative? Line B? b. Calculate the slope of Line A. Write the equation describing the line in the form Y...
-
Select a service brand you consider to be outstanding. Explain why you think it is outstanding. Also explore any weaknesses of this brand. You should select an organization you are familiar with.
-
Why are standards necessary to control the process of updating a website? Give three examples of different aspects of a website that need to be controlled.
-
Choose an industry you are familiar with (such as cell phone services, credit cards, or music streaming) and create a perceptual map showing the competitive positions of different service providers...
-
You have been appointed manager of a website for a car manufacturer and have been asked to refine the existing online measurement and improvement programme. Explain, in detail, the steps you would...
-
Most companies collect data about digital marketing activities, but few derive much value from it. Discuss possible reasons for this assertion.
-
What is the pressure exerted by a beam of light on a perfect mirror from which it reflects at normal (perpendicular) incidence? Generalize this to light incident at an angle to the normal on an...
-
A line l passes through the points with coordinates (0, 5) and (6, 7). a. Find the gradient of the line. b. Find an equation of the line in the form ax + by + c = 0.
-
List the symmetry elements for (a) H 2 O (bent). (b) CO 2 (linear).
-
Find the 3 by 3 matrix that is equivalent in its action to each of the symmetry operators: (a) C 8(x) . (b) S 6(x) .
-
List the symmetry elements of a uniform cube centered at the origin with its faces perpendicular to the coordinate axes.
-
J is going to receive a 30-year annuity of 8,500 and L is going to receive perpetuity of 8,500. If the appropriate interest rate is 6%, how much more is L's cash flow worth?
-
. Assume that over the past 88 years, U.S. Treasury bills had an average return of 3.5% as compared to 6.1% on long-term government bonds. What was the average risk premium on the long-term...
-
The following data were gathered to use in reconciling the bank account of Bradford Company: Balance per bank $ 18,050 Balance per company records 10,040 Bank service charges 50 Deposit in transit...

Study smarter with the SolutionInn App


