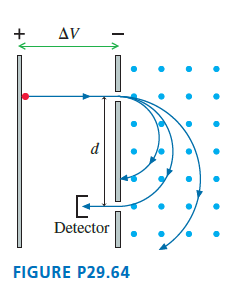
FIGURE P29.64 shows a mass spectrometer, an analytical instrument used to identify the various molecules in a
Question:
See Exercise 29 for atomic masses; the mass of the missing electron is less than 0.001 u and is not relevant at this level of precision. Although N2+ and CO+ both have a nominal molecular mass of 28, they are easily distinguished by virtue of their slightly different accelerating voltages. Use the following constants: 1 u = 1.6605 × 10-27 kg, e = 1.6022 × 10-19 C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:





