In a chemical reaction, one unit of compound Y and one unit of compound Z are converted
Question:
In a chemical reaction, one unit of compound Y and one unit of compound Z are converted into a single unit of compound X. Let x be the amount of compound X formed. The rate of formation of X is proportional to the product of the amounts of unconverted compounds Y and Z. So, dx/dt = k(y0 - x)(z0 - x), where y0 and z0 are the initial amounts of compounds Y and Z. From this equation, you obtain
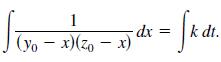
(a) Solve for x as a function of t.(b) Use the result of part (a) to find x as t ∞ for(1) y00,
(2) y0 > z0,
(3) y0 = z0.

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Calculus Of A Single Variable
ISBN: 9781337275361
11th Edition
Authors: Ron Larson, Bruce H. Edwards
Question Posted:





