A solution with an ionic strength of 0.10 M containing 0.010 0 M phenylhydrazine has a pH
Question:
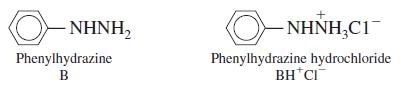
A solution with an ionic strength of 0.10 M containing 0.010 0 M phenylhydrazine has a pH of 8.13. Using activity coefficients correctly, find pKa for the phenylhydrazinium ion found in phenylhydrazine hydrochloride. Assume that λBH+ = 0.80.
Transcribed Image Text:
ΝΗΝΗ, O- NHNH,C1- Phenylhydrazine Phenylhydrazine hydrochloride BH*CI B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (16 reviews)
The chemical reaction between phenylhydrazine B an...View the full answer

Answered By

Tamil Elakkiya Rajendran
I'm currently involved in the research in the field of Biothermodynamics, Metabolic pathway analysis and computational Biology. I always prefer to share my knowledge whatever I have learnt through my degree whenever time permits.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Effect of ionic strength on pKa. Ka for the H2PO4- /HPO24- buffer is If you mix a 1:1 mole ratio of H2PO-4 and HPO42- at 0 ionic strength, the pH is 7.20. Using activity coefficients from Table 7-1,...
-
At 25C, k = 1.55 dm6 mol-2 min-1 at an ionic strength of 0.0241 for a reaction in which the rate-determining step involves the encounter of two singly charged cautions. Use the D cbyc-Huckcl Limiting...
-
When 5.00 mL of 0.1032 M NaOH were added to 0.1123 g of alanine (FM 89.093) in 100.0 mL of 0.10 M KNO3, the measured pH was 9.57. Use activity coefficients to find pK2 for alanine. Consider the ionic...
-
Explain why learning reduces the effective marginal cost of production. If firms set prices in proportion to their marginal costs, as suggested by the Economics Primer, how can learning firms ever...
-
Give an example of a Code provision with the limiting language For purposes of this Section. How does it affect the application of the provision you found? (Use a provision other than the example...
-
How does the radius of the earths core compare with the total radius of the earth?
-
Describe the relationship between dynamic modeling, behavioral modeling, and structural modeling.
-
Kara owns 35% of the KLM Partnership and 45% of the KTV Partnership. Lynn owns 20% of KLM and 3% of KTV. Maura, Karas daughter, owns 15% of KTV. No other partners own an interest in both partnerships...
-
Jargon Jen has five million shares outstanding, generates free cash flows of $12 million each year and has a cost of capital of 6%. It also has $3 million of cash on hand. Jargon Jen wants to decide...
-
Sonora County is located in northern California and is known for its wine country and rugged Pacific coast line. Sonora is a rural county with only one major city, Santa Rita, which has a population...
-
(a) Find the pH of a solution prepared by dissolving 1.00 g of glycine amide hydrochloride (Table 8-2) plus 1.00 g of glycine amide in 0.100 L. (b) How many grams of glycine amide should be added to...
-
Use the Goal Seek spreadsheet at the end of the chapter to find the pH of 1.00 L of solution containing 0.030 mol HA (pK a = 2.50) and 0.015 mol NaA. What would the pH be with the approximations [HA]...
-
Nearly all individuals and organizations are subject to pressures and can rationalize. (True/False)
-
Compare and contrast how your local grocery would position the pre-made meal area of its store if it were trying to excel on performance, price, or relational value. What value proposition do you...
-
The following advice is offered on the website of a company designing and making wedding cakes to order. Costs involved in making a wedding cake: Wedding cakes come in all shapes, sizes and price...
-
How is a production function affected by the invention of a new process related to it? Can this change result in lower prices to the consumer? What do you think? Do improvements in technological...
-
What types of pay scheme may be found?
-
How are direct and indirect materials costs distinguished?
-
Use the TELUS Corporation (TELUS) financial statements on My Accounting Lab to answer the following questions: 1. Give the breakdown of TELUS's current liabilities at December 31, 2013. 2. TELUS has...
-
Carlton Stokes owns and operates a car-detailing business named SuperShine & Detailing. For $150, Carltons business will hand wash and wax customers cars, vacuum the interior, and thoroughly clean...
-
The wind power output per unit area swept by the rotor is 2.4 kW/m 2 . Convert this quantity to the dimensions of hp/ft 2 .
-
A world-class runner can run half a mile in a time of 1 min and 45 s. What is the runners average speed in m/s?
-
One U.S. gallon is equivalent to 0.1337 ft 3 , 1 ft is equivalent to 0.3048 m, and 1000 L are equivalent to 1 m 3 . By using those definitions, determine the conversion factor between gallons and...
-
Shahid Pakistan Limited (SPL) is engaged in the production of three products: J, K and L. Following is the extract from its latest annual management accounts: Description J Products K Total L Units...
-
John Company produces hats and sells them for $100 each. His cost to produce the hats are: DM 20 per unit DL 30 per unit VMOH 10 per unit FMOH 40,000 Selling expenses are $5 per unit and are all...
-
John Company produces hats and sells them for $100 each. His cost to produce the hats are: DM 20 per unit DL 30 per unit VMOH 10 per unit FMOH 40,000 Selling expenses are $5 per unit and are all...

Study smarter with the SolutionInn App


