Nanoparticles with a gold core and organothiol outer shell with well-defined stoichiometries Aux(SR)yAu x (SR) y i
Question:
Nanoparticles with a gold core and organothiol outer shell with well-defined stoichiometries Aux(SR)yAux(SR)y i can be prepared and isolated.
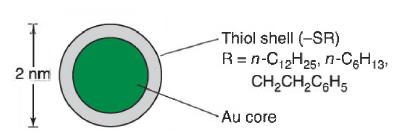
a. The MALDI-time-of-flight mass spectrum of the nanoparticle with R=CH2CH2C6H5 R=CH2CH2C6H5 exhibits a broad molecular ion at m/z 76.3 kDa. m/z 76.3 kDa. Electrospray ionization gives a series of sharp peaks at m/z 38 152.7 m/z 38 152.7 (charge=+2),(charge =+2), 25 433.3 (+3),25 433.3 (+3), 19 075.2 (+4),19 075.2 (+4), and 15 260.4 (+5). 15 260.4 (+5). Find the average molecular mass of Aux(SCH2CH2C6H5)y Aux(SCH2CH2C6H5)y from the four peaks.
b. In another electrospray experiment, the (z=-3) (z=-3) ions for three different thiol shells were found at m/z 27 243 (R=n-C12H25), m/z 27 243 (R=n-C12H25) 25 440 25 440(R=CH2CH2C6H5),(R=CH2CH2C6H5), and 24 8762 24 8762 (R=n-C6H13).(R=n-C6H13)Compute the mass of the molecular ion for the three Aux(SR)y Aux(SR)y nanoparticles. Assuming that xx is constant, find the value of yy for each nanoparticle from the difference in molecular mass between the molecular ions. From the value of yy find the value of xx. Note that AuAu is monoisotopic with a mass of 196.966 6.196.966 6.
Step by Step Answer:

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy





