One glass-membrane sodium ion-selective electrode has a selectivity coefficient . When this electrode was immersed in 1.00
Question:
One glass-membrane sodium ion-selective electrode has a selectivity coefficient . When this electrode was immersed in 1.00 mM NaCl at pH 8.00, a potential of -38 mV (versus S.C.E.) was recorded.
(a) Neglecting activity coefficients, calculate the potential with Equation 14-10 if the electrode were immersed in 5.00 mM NaCl at pH 8.00.
(b) What would the potential be for 1.00 mM NaCl at pH 3.87? You can see that pH is a critical variable for the sodium electrode.
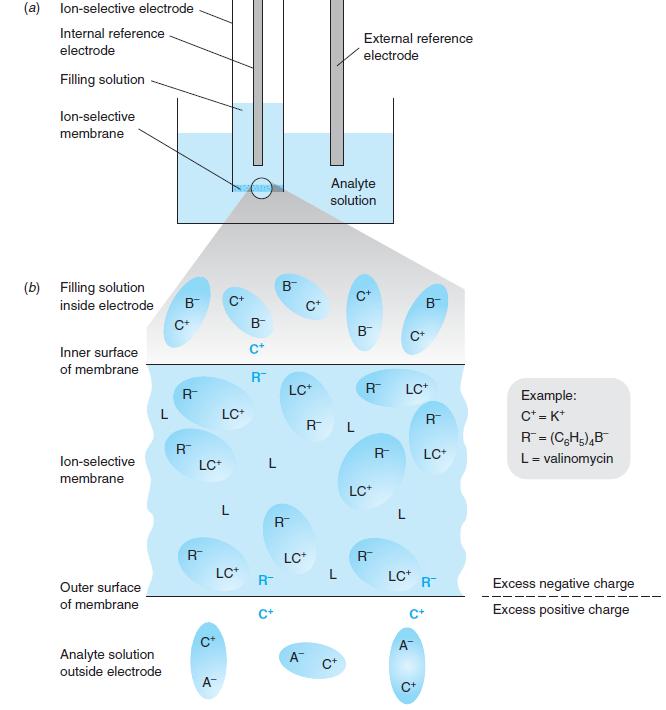
Figure 14-10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





