Question: 1. Resonance (12 points) Draw two additional resonance structures for each molecule below. CH2-CH=CH-Br @CH2-CH=CH-CN 2. Nomenclature (9 points) Draw structures for each name below.
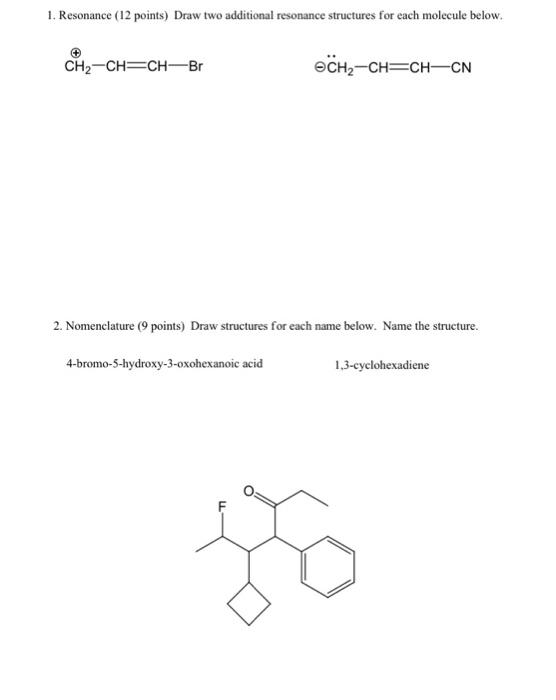
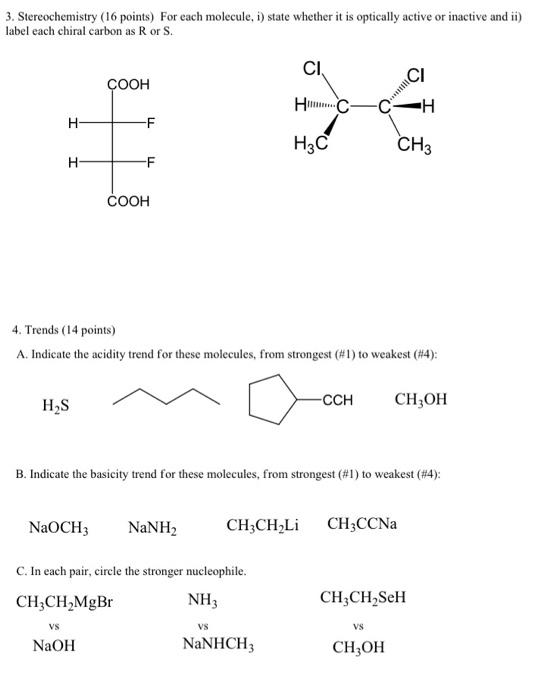
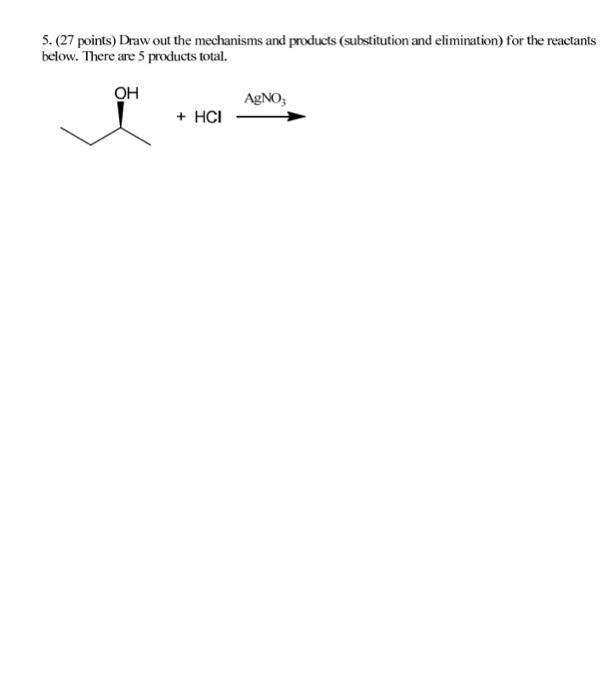
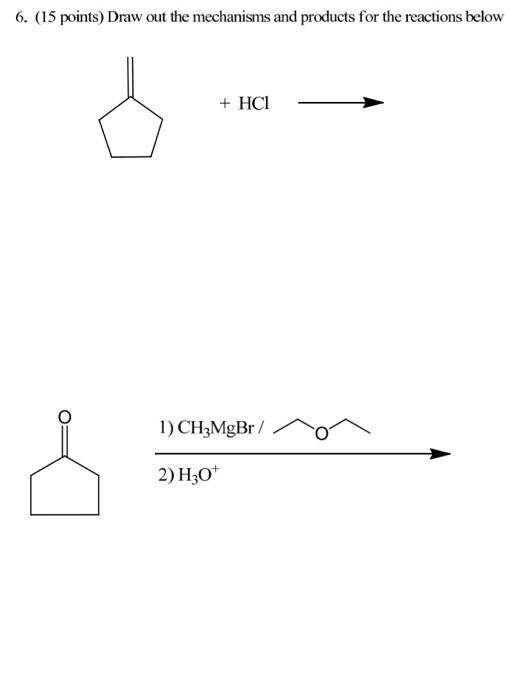
1. Resonance (12 points) Draw two additional resonance structures for each molecule below. CH2-CH=CH-Br @CH2-CH=CH-CN 2. Nomenclature (9 points) Draw structures for each name below. Name the structure. 4-bromo-5-hydroxy-3-oxohexanoic acid 1.3-cyclohexadiene go 3. Stereochemistry (16 points) For each molecule, i) state whether it is optically active or inactive and ii) label each chiral carbon as Ror S. CI CI COOH H H -F H3C CH3 H -F COOH 4. Trends (14 points) A. Indicate the acidity trend for these molecules, from strongest (#1) to weakest (#4): HS -CCH , B. Indicate the basicity trend for these molecules, from strongest (#1) to weakest (#4): NaOCH; NaNH2 CHCH Li CH3CCNa C. In each pair, circle the stronger nucleophile. CH2CH MgBr NH CH3CH SEH VS VS VS NaOH NaNHCH, CH3OH 5. (27 points) Draw out the mechanisms and products (substitution and elimination) for the reactants below. There are 5 products total. OH AgNO3 + HCI 6. (15 points) Draw out the mechanisms and products for the reactions below + HCI 1) CH3MgBr / 2) H307
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


