Question: answer all of these questions please 1. Give the IUPAC name for the following structures: a) Br ity b) b) 2. Draw the following structures
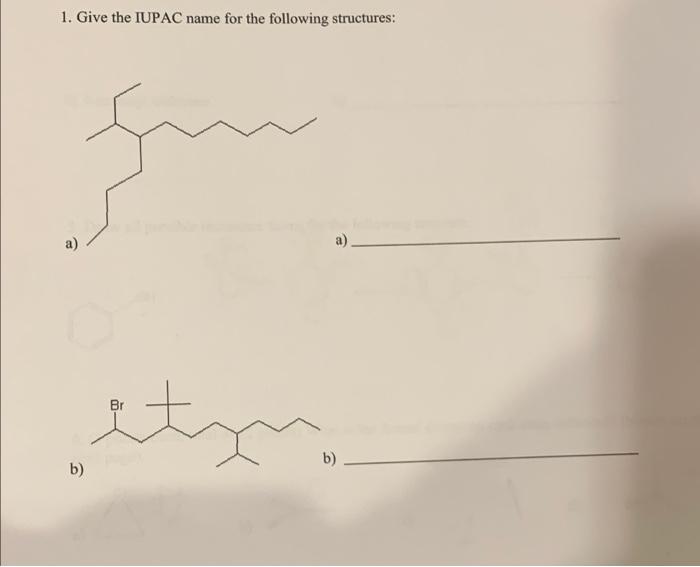
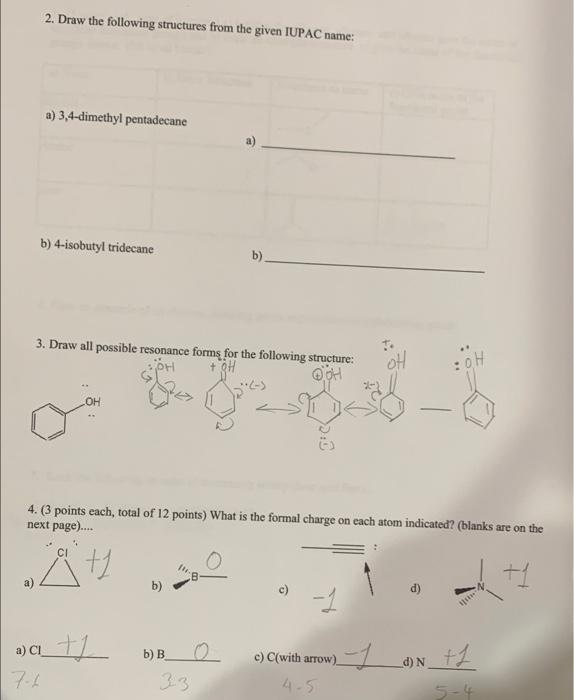
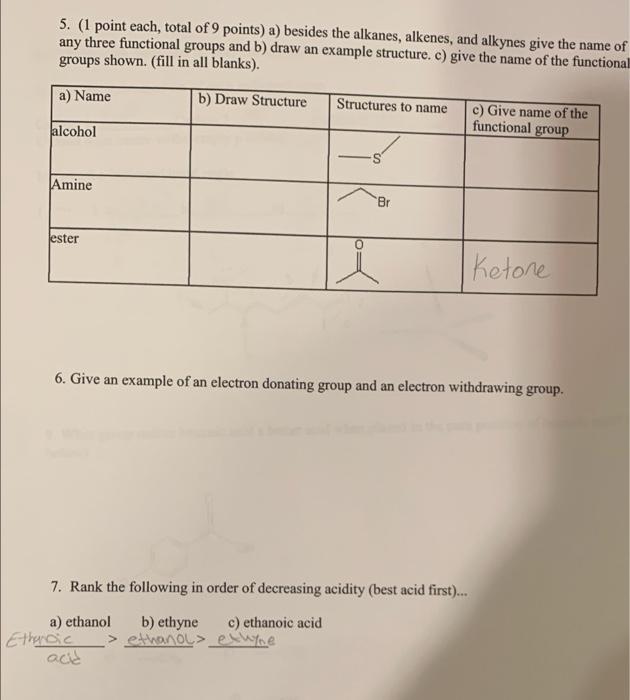
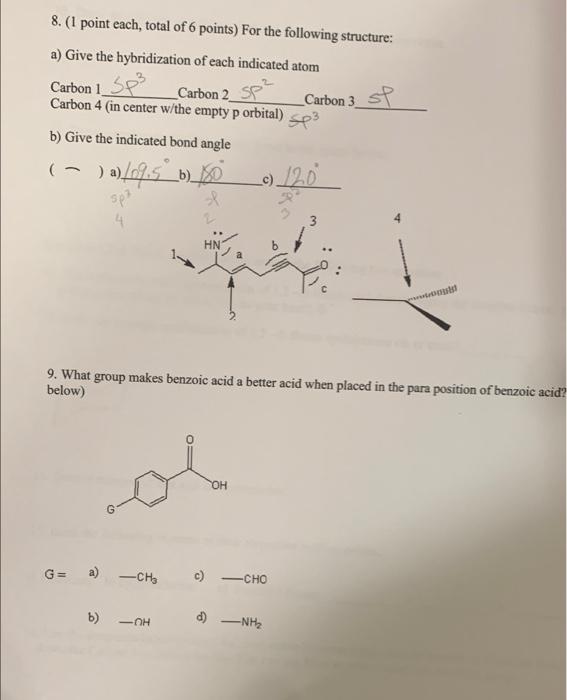

1. Give the IUPAC name for the following structures: a) Br ity b) b) 2. Draw the following structures from the given IUPAC name: a) 3,4-dimethyl pentadecane b) 4-isobutyl tridecane b) 3. Draw all possible resonance forms for the following structure: + 9H oH :0H OH OH 4. (3 points each, total of 12 points) What is the formal charge on each atom indicated? (blanks are on the next page).... + 8 t b) a) a t1 b) B 23 3-7 an tl d) N 7.4 c) C(with arrow) 4-5 5-4 5. (1 point each, total of 9 points) a) besides the alkanes, alkenes, and alkynes give the name of any three functional groups and b) draw an example structure. c) give the name of the functional groups shown. (fill in all blanks). a) Name b) Draw Structure Structures to name c) Give name of the functional group alcohol Amine Br ester Ketone 6. Give an example of an electron donating group and an electron withdrawing group. 7. Rank the following in order of decreasing acidity (best acid first)... a) ethanol b) ethyne c) ethanoic acid Ethnic > ethanol ethype acd 2 Carbon 1 SP 8. (1 point each, total of 6 points) For the following structure: a) Give the hybridization of each indicated atom Carbon 2_sp Carbon 3 st Carbon 4 (in center w/the empty p orbital) sp3 b) Give the indicated bond angle (-)a)[0%.5_b)_10 sp? HN 9. What group makes benzoic acid a better acid when placed in the para position of benzoic acid? below) G G= a) - CH c) CHO b) -OH d) -NH2 10. Draw any isomer of heptane that has six carbons in the main chain. 11. Draw a molecule that has a polar bond in the space below. Consider 1,2 di fluoro cyclohexane to answer the following two questions. 12. Draw the most stable conformation of 1,2-di fluoro cyclohexane using the template below. (If you are drawing everything by hand, just do your best!) 13. Is the most stable conformation of 1,2-di fluoro cyclohexane cis or trans? 14. Draw a compound that will be hydrophobic. 15. Rank the following in terms of their respective melting points. a) Undecane b) cycloundecanec) 3-methyl decane 16. Use the templates below to draw the most stable Newman Projection for hexane as if you are looking down the Carbon 3 - Carbon 4 bond. You can use Et as an abbreviation for the ethyl group
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


