Question: 1. Which compound has a higher boiling point? a. (i) pentan-1,5-diol or (ii) hexan-1-ol b. (i) hexan-2-ol or (i) hexane-1,5-diol c. (i) hexan-2-ol or
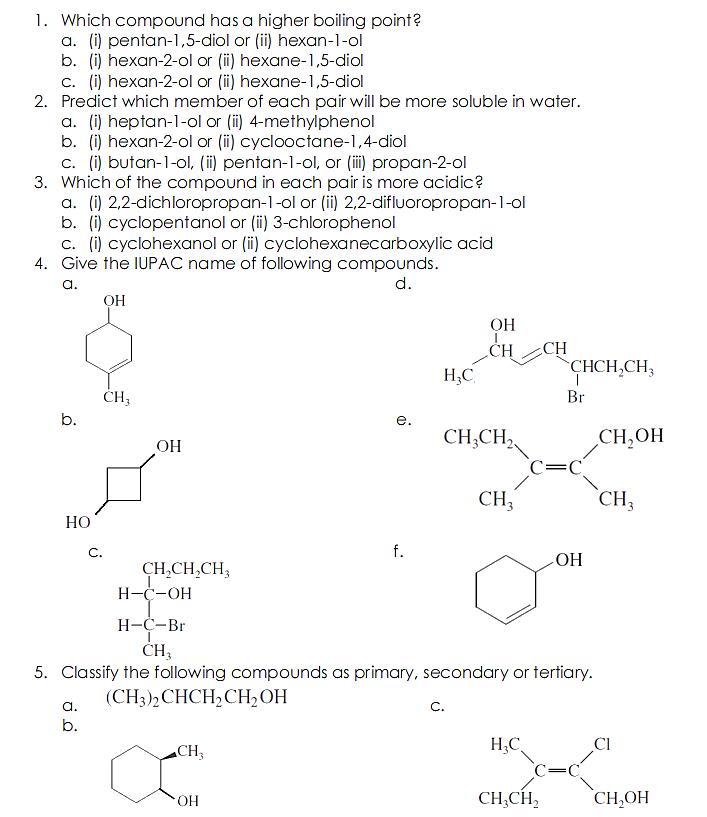
1. Which compound has a higher boiling point? a. (i) pentan-1,5-diol or (ii) hexan-1-ol b. (i) hexan-2-ol or (i) hexane-1,5-diol c. (i) hexan-2-ol or (ii) hexane-1,5-diol 2. Predict which member of each pair will be more soluble in water. a. (i) heptan-1-ol or (i) 4-methylphenol b. (i) hexan-2-ol or (i) cyclooctane-1,4-diol c. (i) butan-1-0ol, (ii) pentan-1-ol, or (ii) propan-2-ol 3. Which of the compound in each pair is more acidic? a. (i) 2,2-dichloropropan-1-ol or (ii) 2,2-difluoropropan-1-ol b. (i) cyclopentanol or (ii) 3-chlorophenol c. (i) cyclohexanol or (i) cyclohexanecarboxylic acid 4. Give the IUPAC name of following compounds. a. d. CH CH CHCH,CH, H,C H; Br b. . CH,OH C=C CH;CH, CH, CH HO C. f. CH,CH,CH3 H-C-OH --Br CH, 5. Classify the following compounds as primary, secondary or tertiary. (CH3)2CHCH2CH2OH a. C. b. CH3 H;C CI CH,CH, CH,OH HO.
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
a Pentan 15 diol hexan10l This is because Pentan15 diol has a higher extent of intemolecular Hydroge... View full answer

Get step-by-step solutions from verified subject matter experts


