Question: a Try Again Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is
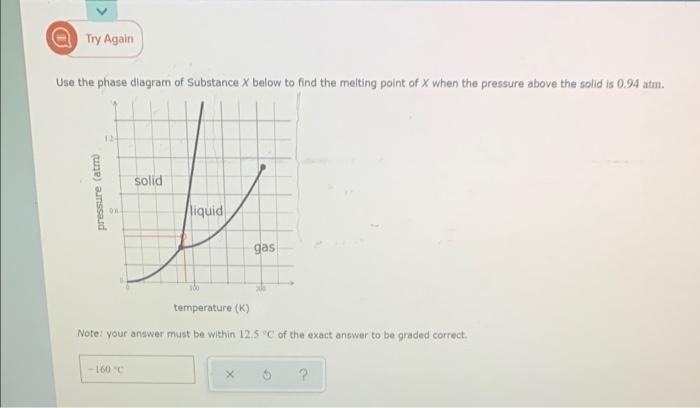
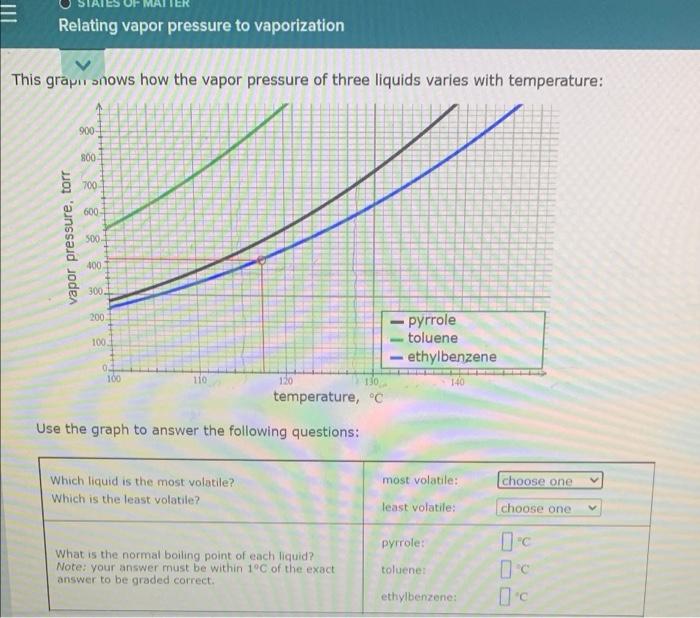
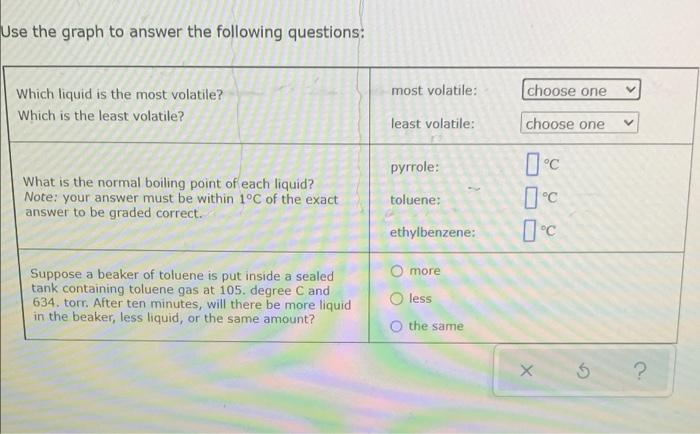
a Try Again Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 0.94 atr. 12 solid pressure (atm liquid gas temperature (K) Note: your answer must be within 12.5C of the exact answer to be graded correct -100c 5 2 E Relating vapor pressure to vaporization This grape snows how the vapor pressure of three liquids varies with temperature: 900 800 700 600 vapor pressure, torr 500 400 300 200 100 pyrrole toluene -ethylbenzene 0 100 110 TO 120 130 temperature, C Use the graph to answer the following questions: choose one Which liquid is the most volatile? Which is the least volatile? most volatile: least volatile: choose one Pyrrole: Ic What is the normal boiling point of each liquid? Note: your answer must be within 1C of the exact answer to be graded correct. toluene: ethylbenzene: Ic Use the graph to answer the following questions: most volatile: choose one Which liquid is the most volatile? Which is the least volatile? least volatile: choose one pyrrole: What is the normal boiling point of each liquid? Note: your answer must be within 1C of the exact answer to be graded correct. toluene: ethylbenzene: O more Suppose a beaker of toluene is put inside a sealed tank containing toluene gas at 105. degree C and 634. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? less the same 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


