Question: PLEASE HELP SOLVE ASAP PLEASEEE SOLVE ALL PLEASE Write your answer on a blank sheet of paper, you must show all your calculation work clearly
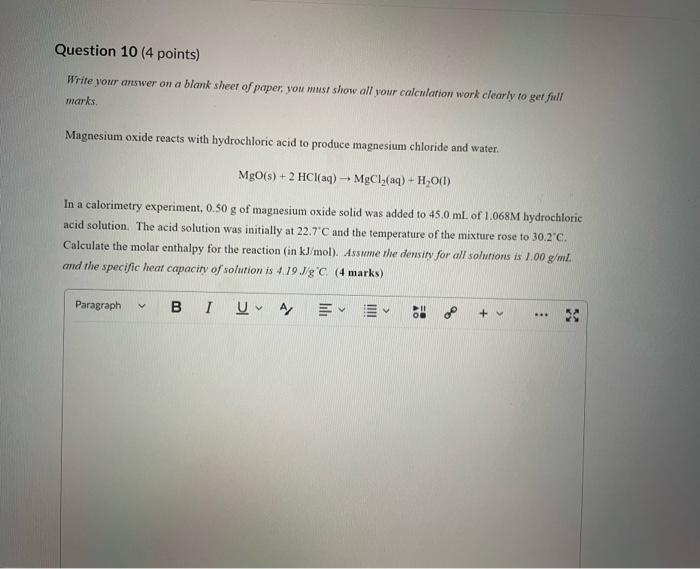
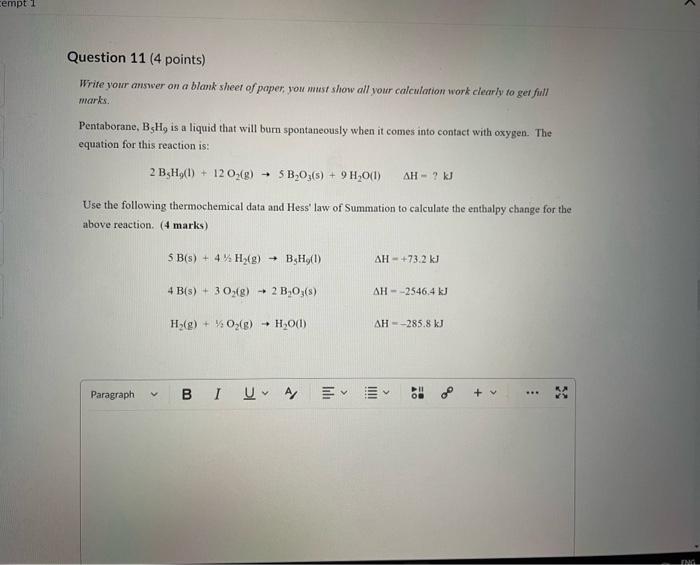
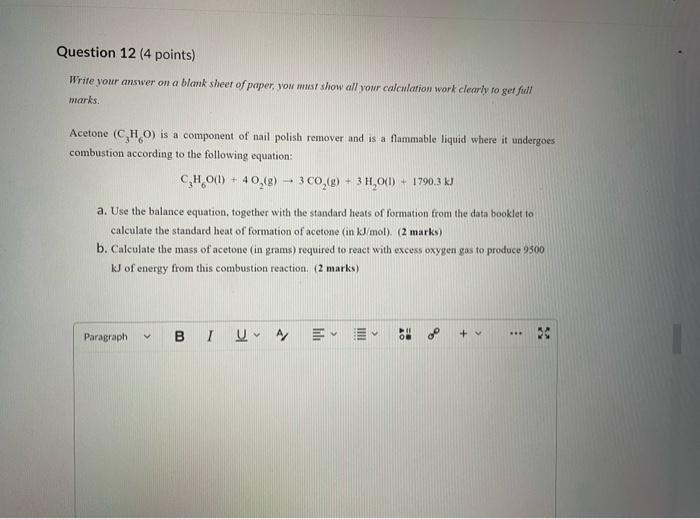
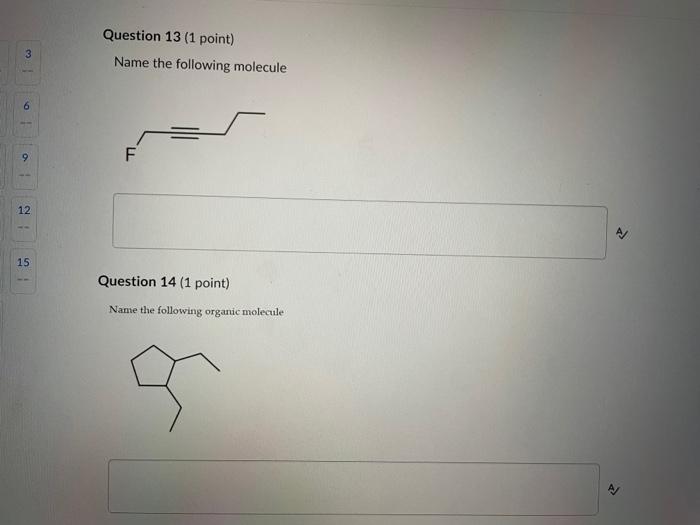
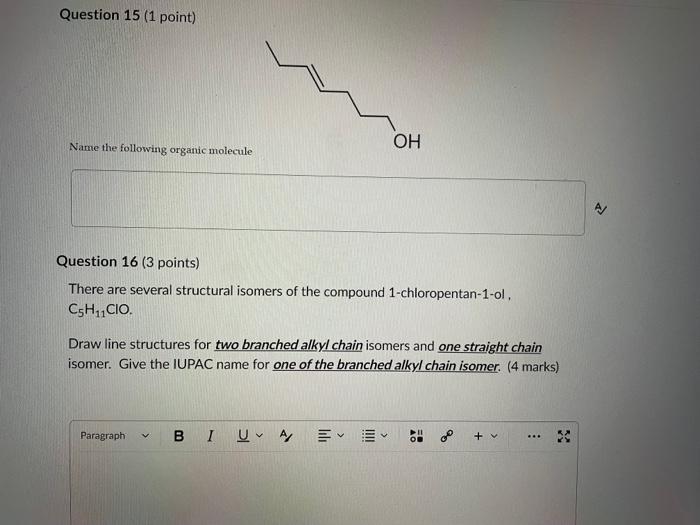
Write your answer on a blank sheet of paper, you must show all your calculation work clearly to get full marks. Magnesium oxide reacts with hydrochloric acid to produce magnesium chloride and water. MgO(s)+2HCl(aq)MgCl2(aq)+H2O(l) In a calorimetry experiment, 0.50g of magnesium oxide solid was added to 45.0mL of 1.068M hydrochloric acid solution. The acid solution was initially at 22.7C and the temperature of the mixture rose to 30.2C. Calculate the molar enthalpy for the reaction (in kJ/mol ). Asstame the density for all solutions is /.00g/mL. and the specific heat capacity of solution is 4.19.J/gC. (4 marks) Write your answer on a blank sheer of paper, you mast show all your calculation work clearly to get full marks. Pentaborane, B5H9 is a liquid that will burn spontaneously when it comes into contact with oxygen. The equation for this reaction is: 2B5H9(1)+12O2(g)5B2O3(5)+9H2O(1)AH=?kJ Use the following thermochemical data and Hess' law of Summation to calculate the enthalpy change for the above reaction. ( 4 marks) 5B(s)+4K2H2(g)B5H9(l)4B(s)+3O2(g)2B2O3(s)H2(g)+1/O2(g)H2O(l)H=+73.2kJH=2546.4kJH=285.8kJ Write your answer on a blank sheer of paper, you must show all your calculation work cleariy ro get full marks. Acetone (C3H6O) is a component of nail polish remover and is a flammable liquid where it undergoes. combustion according to the following equation: C3H6O(l)+4O2(g)3CO2(g)+3H2O(I)+1790.3kJ a. Use the balance equation, together with the standard heats of formation from the data booklet to calculate the standard heat of formation of acetone (in kJ/mol). ( 2 marks) b. Calculate the mass of acetone (in grams) required to react with excess oxygen gas to produce 9500 kJ of energy from this combustion reaction. ( 2 marks) Question 13 (1 point) Name the following molecule Question 14 (1 point) Nante the following organic molectule Name the following organic molecu Question 16 ( 3 points) There are several structural isomers of the compound 1-chloropentan-1-ol, C5H11ClO Draw line structures for two branched alkyl chain isomers and one straight chain isomer. Give the IUPAC name for one of the branched alkyl chain isomer. (4 marks) Draw the line structures to represent the reactants and products in the formation of butyl propanoate. Give the IUPAC name of the reactants. ( 3 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


