Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. A 25.0 g sample of nitrogen gas, N, has a volume of 50.0 L and a pressure of 635 mm Hg. What is



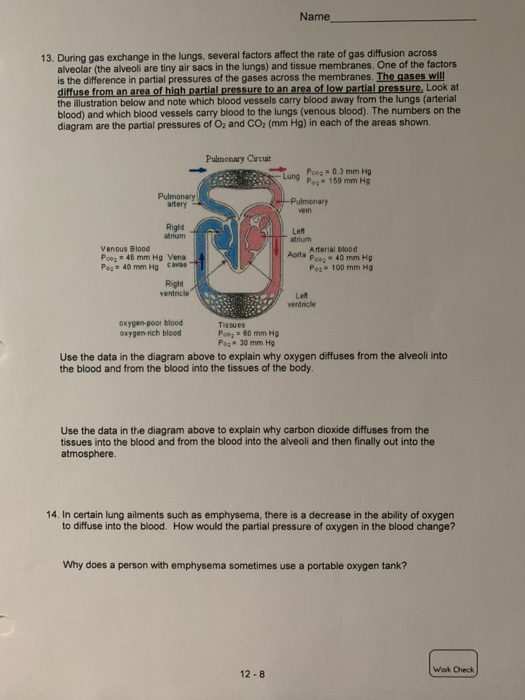
2. A 25.0 g sample of nitrogen gas, N, has a volume of 50.0 L and a pressure of 635 mm Hg. What is the temperature of the gas? Work Check 3. How many molecules of gas will fill a 30.0 gallon trash bag at 25 C and 760. mm Hg? (1 L = 1.06 qts; 1 gal = 4 qts) 4. Aerosol containers can be dangerous if they are heated because they can explode. Suppose a container of hair spray with a pressure of 4.0 atm at a room temperature of 25 C is thrown into a fire. a) If the temperature of the gas inside the aerosol can reaches 402 C, what will the pressure be? b) The aerosol container may explode if the pressure inside exceeds 8.0 atm. Would you expect it to explode? 12-5 Wok Check Name 5. A volume of 500. mL of air on a cold winter day at 5 C is inhaled into the lungs. The body temperature is 37 C. What will be the new volume of the air in the lungs? Assume the pressure remains constant at 760 mm Hg. 6. The volume of air in a person's lungs is 615 mL at a pressure of 760. mm Hg. Inhalation occurs as the pressure in the lungs drops to 752 mm Hg. To what volume did the lungs expand? 7. A sample of gas containing 1.50 moles of neon has a volume of 8.50 L. What will be the volume of the sample of gas if a leak allows one-half of the neon atoms to escape? 8. A sample containing 4.80 g of oxygen gas, O, has a volume of 15.0 L. Oxygen gas is released until the volume is 10.0 L. How many moles of oxygen are removed? 12-6 C Name 9. What volume will 3.5g of CO occupy at 17 C and 1875 torr? 10. A typical air sample in the lungs contains oxygen at 100 mm Hg, nitrogen at 573 mm Hg, carbon dioxide at 40 mm Hg, and water vapor at 47 mm Hg. Why are these pressures called partial pressures? 11. An anesthetic consists of a mixture of cyclopropane gas, CH, and oxygen gas, O. If the mixture has a total pressure of 825 torr, and the partial pressure of the cyclopropane gas is 73 torr, what is the partial pressure of the oxygen in the anesthetic? 12. If 74.5 % of exhaled air is nitrogen, what is the partial pressure of nitrogen in exhaled air. Assume that the total pressure of exhaled air is 760. mm Hg. 12-7 Work Check 13. During gas exchange in the lungs, several factors affect the rate of gas diffusion across alveolar (the alveoli are tiny air sacs in the lungs) and tissue membranes. One of the factors is the difference in partial pressures of the gases across the membranes. The gases will diffuse from an area of high partial pressure to an area of low partial pressure, Look at the illustration below and note which blood vessels carry blood away from the lungs (arterial blood) and which blood vessels carry blood to the lungs (venous blood). The numbers on the diagram are the partial pressures of O and CO (mm Hg) in each of the areas shown. Pulmonary artery Right atrium Venous Blood Poog 46 mm Hg Vena Po: 40 mm Hg cavae Right ventricle oxygen-poor blood oxygen-rich blood Pulmonary Circuit Tissues Pco= 60 mm Hg Po: 30 mm Hg Name Lung Poog = 0.3 mm Hg 159 mm Hg Pog -Pulmonary vein 12-8 Left atrium Arterial blood 40 mm Hg Poz 100 mm Hg Aorta Po Left ventricle Use the data in the diagram above to explain why oxygen diffuses from the alveoli into the blood and from the blood into the tissues of the body. Use the data in the diagram above to explain why carbon dioxide diffuses from the tissues into the blood and from the blood into the alveoli and then finally out into the atmosphere. 14. In certain lung ailments such as emphysema, there is a decrease in the ability of oxygen to diffuse into the blood. How would the partial pressure of oxygen in the blood change? Why does a person with emphysema sometimes use a portable oxygen tank? Work Check
Step by Step Solution
★★★★★
3.50 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
2 The temperature of a gas can be calculated using the Ideal Gas Law PV nRT where P is pressure V is volume n is the number of moles R is the ideal gas constant and T is the temperature in kelvin Firs...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


