Answered step by step
Verified Expert Solution
Question
1 Approved Answer
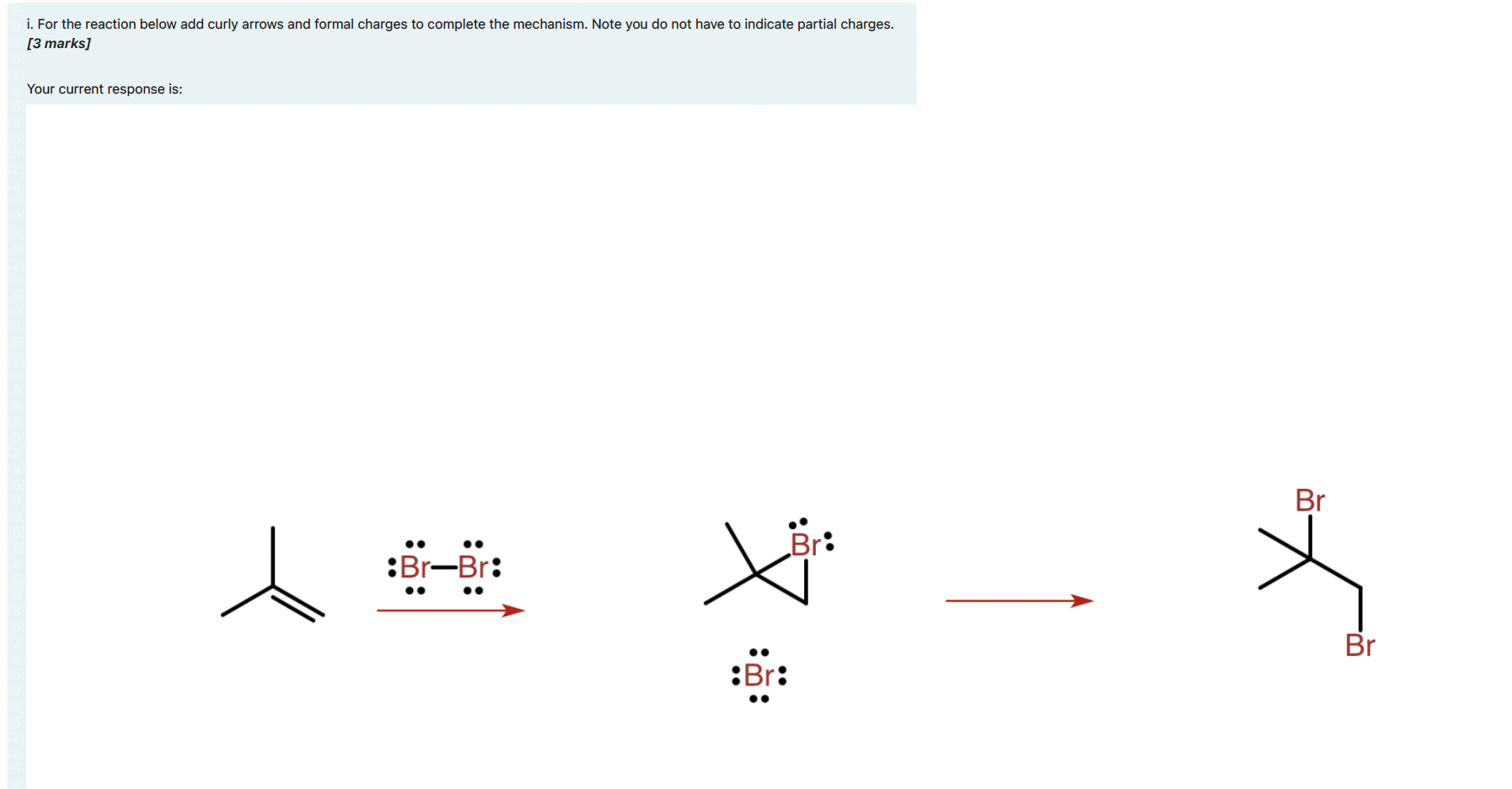
i. For the reaction below add curly arrows and formal charges to complete the mechanism. Note you do not have to indicate partial charges.
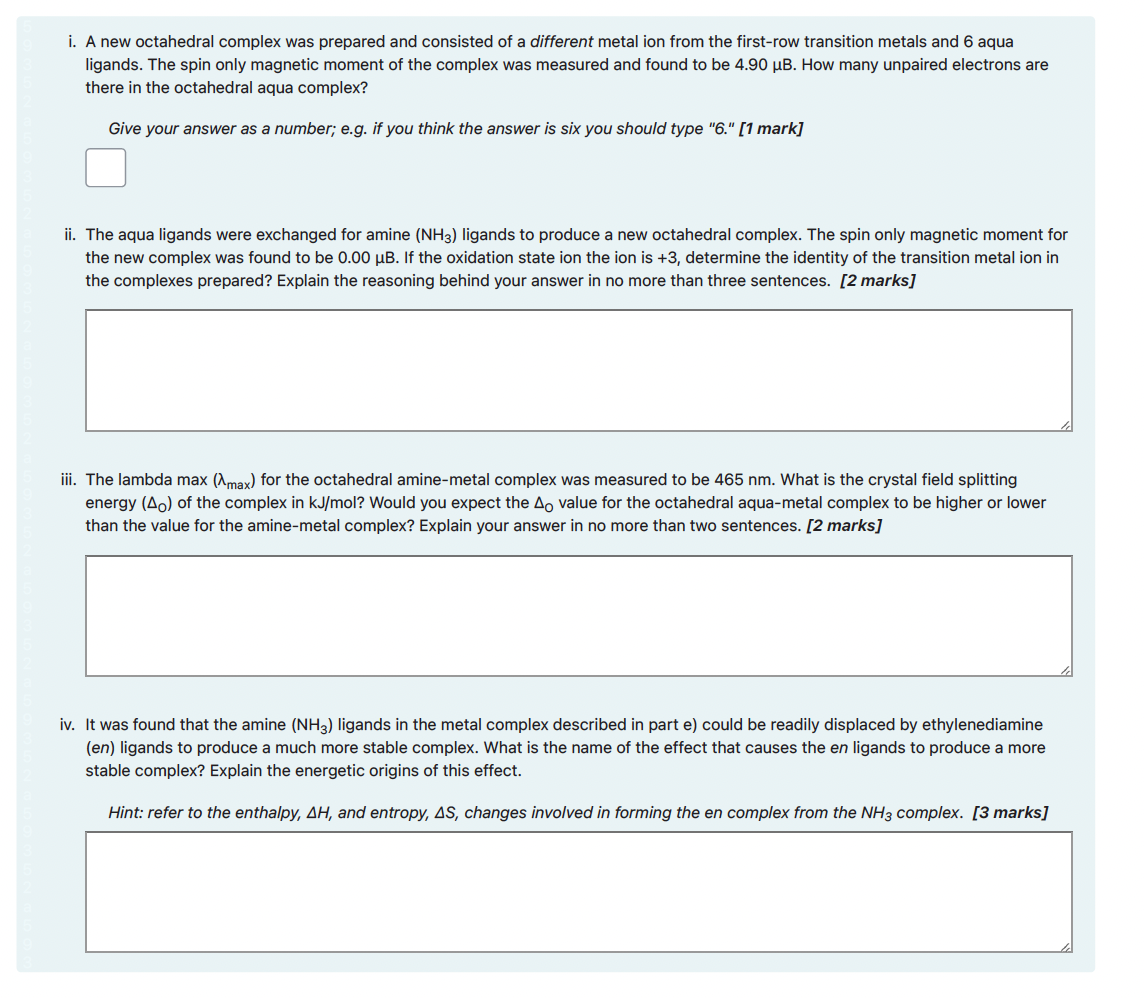
i. For the reaction below add curly arrows and formal charges to complete the mechanism. Note you do not have to indicate partial charges. [3 marks] Your current response is: Br Br: :Br-Br: Br :Br: i. A new octahedral complex was prepared and consisted of a different metal ion from the first-row transition metals and 6 aqua ligands. The spin only magnetic moment of the complex was measured and found to be 4.90 B. How many unpaired electrons are there in the octahedral aqua complex? Give your answer as a number; e.g. if you think the answer is six you should type "6." [1 mark] ii. The aqua ligands were exchanged for amine (NH3) ligands to produce a new octahedral complex. The spin only magnetic moment for the new complex was found to be 0.00 B. If the oxidation state ion the ion is +3, determine the identity of the transition metal ion in the complexes prepared? Explain the reasoning behind your answer in no more than three sentences. [2 marks] iii. The lambda max (Amax) for the octahedral amine-metal complex was measured to be 465 nm. What is the crystal field splitting energy (Ao) of the complex in kJ/mol? Would you expect the Ao value for the octahedral aqua-metal complex to be higher or lower than the value for the amine-metal complex? Explain your answer in no more than two sentences. [2 marks] iv. It was found that the amine (NH3) ligands in the metal complex described in part e) could be readily displaced by ethylenediamine (en) ligands to produce a much more stable complex. What is the name of the effect that causes the en ligands to produce a more stable complex? Explain the energetic origins of this effect. Hint: refer to the enthalpy, AH, and entropy, AS, changes involved in forming the en complex from the NH3 complex. [3 marks]
Step by Step Solution
★★★★★
3.32 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6369586c02829_242295.pdf
180 KBs PDF File
6369586c02829_242295.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started