Question: help please Part A: Nomenclature and Structural Isomers of Hydrocarbons Question I a) What is the molecular formula of the alkane? (1 mark) b) What
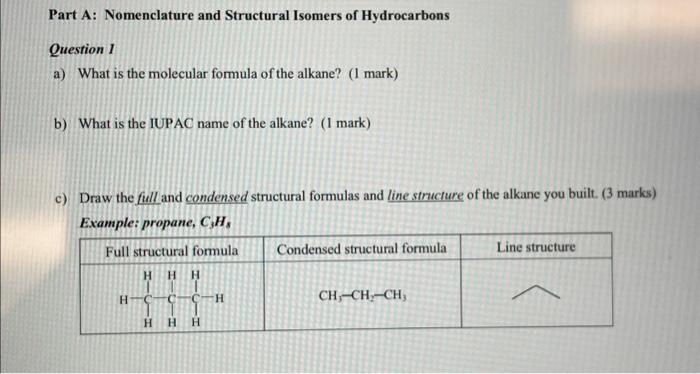



Part A: Nomenclature and Structural Isomers of Hydrocarbons Question I a) What is the molecular formula of the alkane? (1 mark) b) What is the IUPAC name of the alkane? ( 1 mark) c) Draw the full and condensed structural formulas and line structure of the alkane you built. (3 marks) Example: propane, C3Hs a) What is the IUPAC name of the structural isomer you built? ( 1 mark) b) Draw the full and condensed structural formulas and line structure of the isomer. ( 3 marks) Question 3 a) What is the molecular formula of the alkene? (1 mark) b) What is the IUPAC name of the alkene? ( 1 mark) c) Draw the full and condensed structural formulas and line structure of the alkene you built. ( 3 marks) Example: propene, C3H6 Nomrnclatare of Ongenic Canpoundy in 6 d) Other structural isomers can be produced from the alkene you built. Siketch the line michichures of nwo structural branching isomers for the alkene and give the IUPAC names of the isomers. (4 marks) Question 4 a) What is the molecular formula of the alkyne? ( 1 mark) b) What is the IUPAC name of the alkyne? (1 mark) c) Draw the full and condensed structural formulas and line structure of the alkyne you built. ( 3 marks) Example: propyne, C3H2 d) Other structural isomers can be produced from the alkyne you built. Sketch the line structures of boo structural branching isomers for the alkyne and give the IUPAC names of the isomers. (4 marks) PART B: Identifying Functional Group and Naming Organic Compound Activity Question 5 a) Sketch the line structure of the molecule. Circle all the functional groups that you can identify on the line structure and clearly label the name of the functional groups. (2 marks) b) What is the IUPAC name of the organic compound? Give the molecular formula of the compound. ( 1 marks) Question 6 For each organic molecule, - write down the letter and number of each molecules, - sketch the line structure of the molecule, ( 1 mark) - circle all the functional groups present on the line structure, ( 1 mark) - label with the name of the functional groups, ( 1 mark) - write the IUPAC name of the molecule and (1.5 marks) - give the molecular formula of the compound. ( 0.5 mark) Molecule 1: \# =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


