Question: please help with all questions Dinitrogen pentoxide decomposes according to the following equation: 2N2O5(g)2N2(g)+5O2(g)+22.6kJ From this equation, what is the enthalpy of formation of N2O5




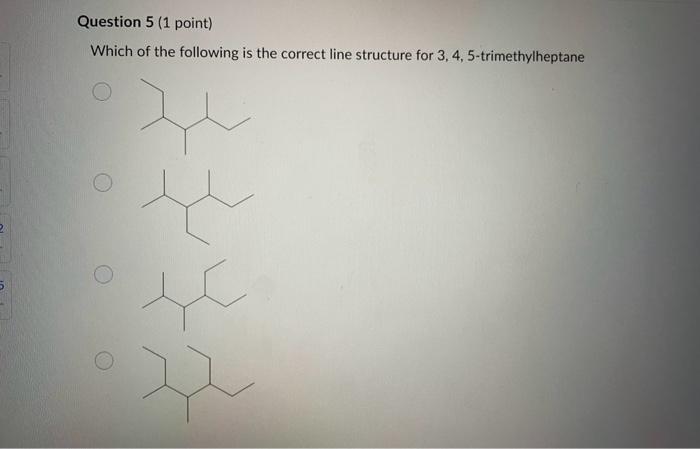
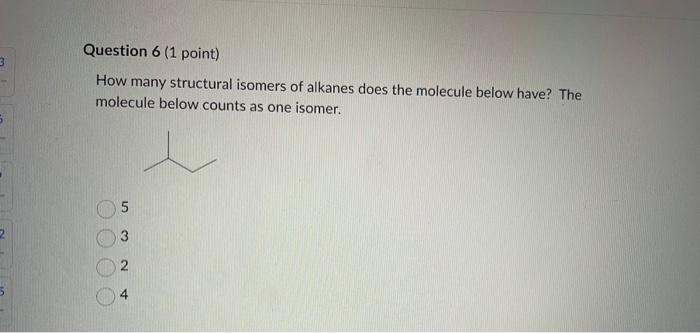
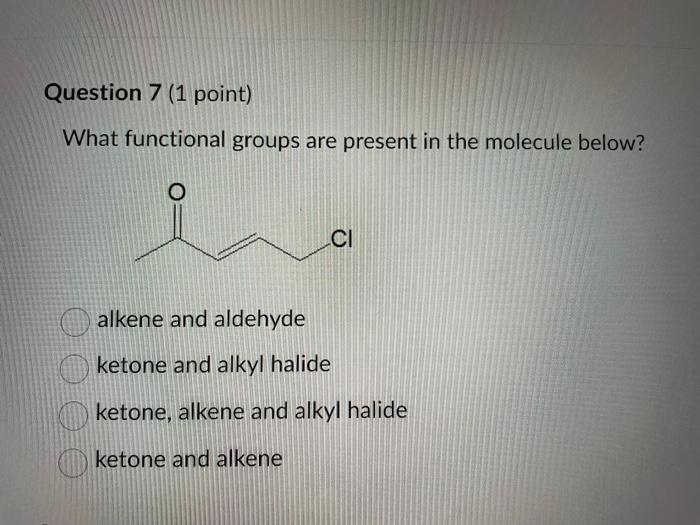
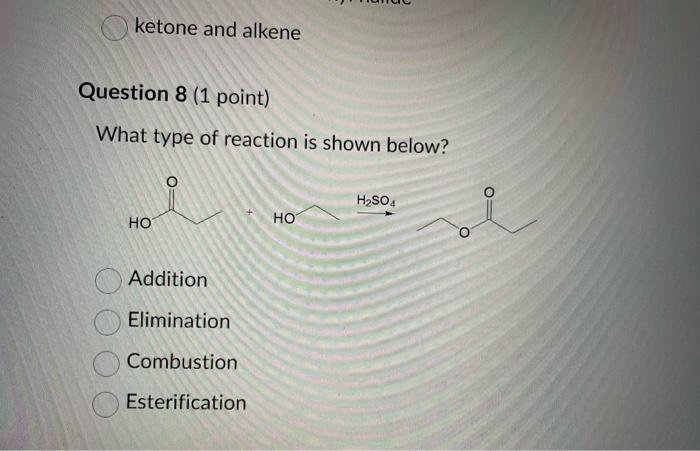
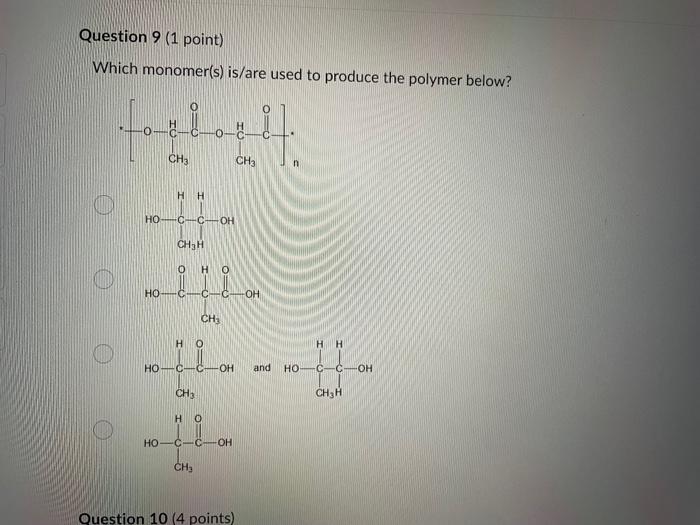
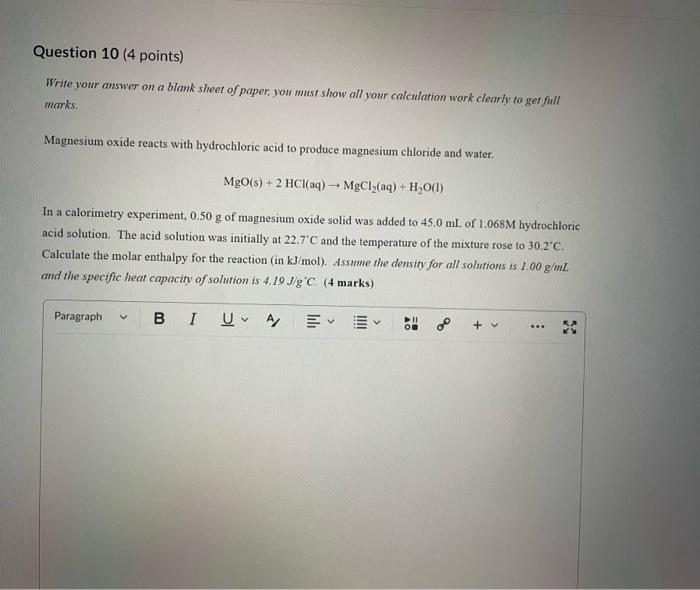
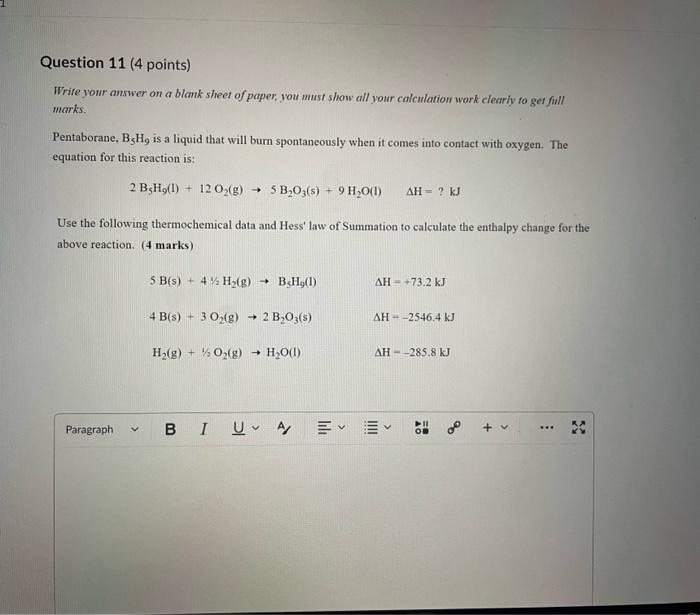
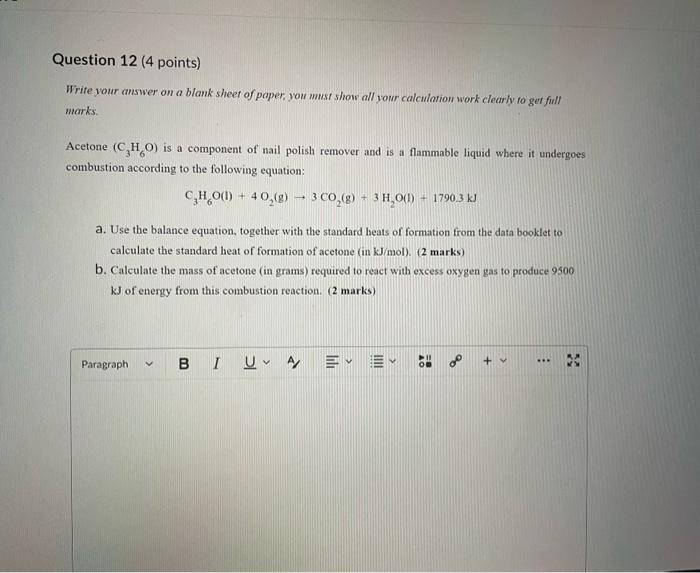
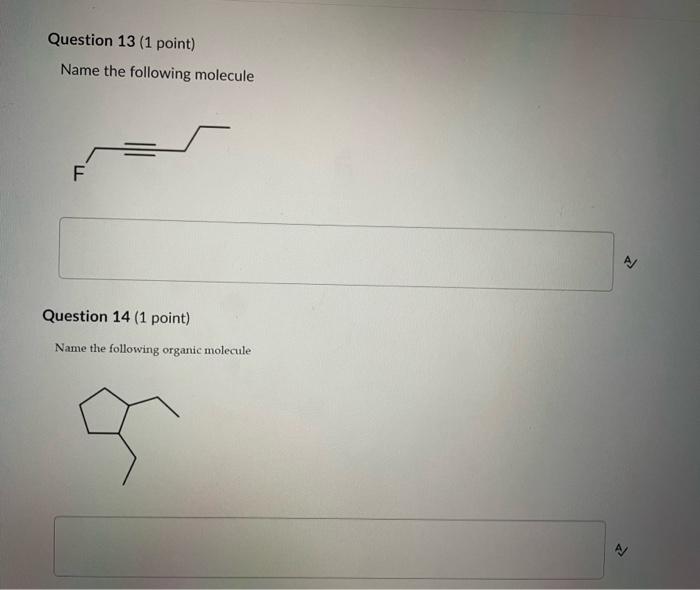
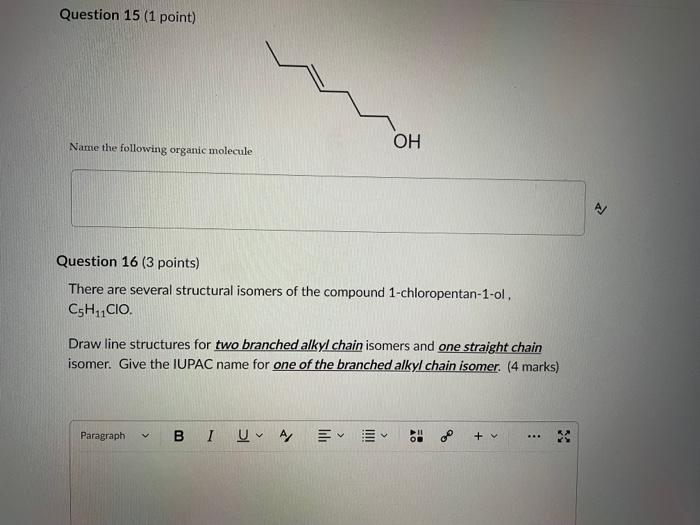
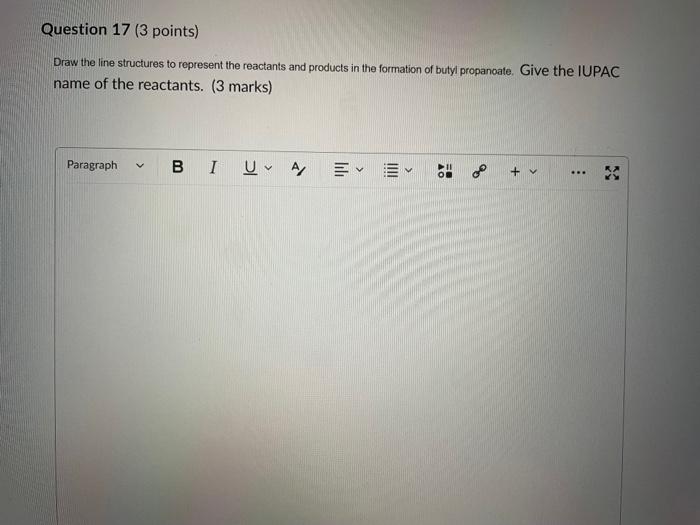
Dinitrogen pentoxide decomposes according to the following equation: 2N2O5(g)2N2(g)+5O2(g)+22.6kJ From this equation, what is the enthalpy of formation of N2O5 gas? 11.3kJ/mol 22.6kJ/mol +45.2kJ/mol +11.3kJ/mol +22.6kJ/mol Question 2 ( 1 point) In the process of sublimation iodine crystals form iodine gas as in the following equation: I2(s)I2(g) Which of the following responses correctly describes this sublimation process? Sign of H is positive: Enthalpy change is exothermic: Potential energy of I2 molecules increases during sublimation. Sign of AH is negative; Enthalpy change is endothermic: Potential energy of I2 molecules decreases during sublimation. Sign of AH is positive; Enthalpy change is exothermic; Potential energy of I2 molecules decreases during sublimation. Sign of AH is negative; Enthalpy change is exothermic; Potential energy of I2 molecules decreases during sublimation. Sign of H is positive; Enthalpy change is endothermic; Potential energy of I2 molecules increases during sublimation. All the following substances have a standard enthalpy of formation value of zero at 25C and 1.00 atn EXCEPT O2(g) Ne(g) Fe(l) F2(g) C(s) The heat of formation of acetylene gas (IUPAC name ethyne), formula C2H2 is +226.7kJ/mol. What does this information tell us about acetylene? Type of hydrocarbon is alkene and the decomposition reaction for acetylene is endothermic. Type of hydrocarbon is alkane and the decomposition reaction for acetylene is endothermic. Type of hydrocarbon is alkyne and the decomposition reaction for acetylene is endothermic. Type of hydrocarbon is alkyne and the decomposition reaction for acetylene is exothermic. Type of hydrocarbon is alkene and the decomposition reaction for acetylene is exothermic. Which of the following is the correct line structure for 3,4,5-trimethylheptane Question 6 (1 point) How many structural isomers of alkanes does the molecule below have? The molecule below counts as one isomer. 5 3 2 4 What functional groups are present in the molecule below? alkene and aldehyde ketone and alkyl halide ketone, alkene and alkyl halide ketone and alkene ketone and alkene Question 8 ( 1 point) What type of reaction is shown below? Addition Elimination Combustion Esterification Which monomer(s) is/are used to produce the polymer below? and Question 10 (4 points) Write your answer on a blank sheet of paper, you must show all your calculation work clearly to get full marks. Magnesium oxide reacts with hydrochloric acid to produce magnesium chloride and water. MgO(s)+2HCl(aq)MgCl2(aq)+H2O(l) In a calorimetry experiment, 0.50g of magnesium oxide solid was added to 45.0mL of 1.068M hydrochloric acid solution. The acid solution was initially at 22.7C and the temperature of the mixture rose to 30.2C. Calculate the molar enthalpy for the reaction (in kJ/mol ). Assume the densiny for all solutions is 1.00g/mL and the specific heat capacity of solution is 4.19J/gC. (4 marks) Wrife your answer on a blank sheet of paper, you must show all your calculation work clearjy to get full marks. Pentaborane, B5H9 is a liquid that will burn spontaneously when it comes into contact with oxygen. The equation for this reaction is: 2B5H9(l)+12O2(g)5B2O3(s)+9H2O(l)H=?kJ Use the following thermochemical data and Hess' law of Summation to calculate the enthalpy change for the above reaction. (4 marks) 5B(s)+4V2H2(g)B5H9(l)4B(s)+3O2(g)2B2O3(s)H2(g)+1/2O2(g)H2O(l)H=+73.2kJH2546.4kJH=285.8kJ Write your answer on a blank sheet of paper, you must show all your calculation work clearly to get full marks. Acetone (C3H6O) is a component of nail polish remover and is a flammable liquid where it undergoes combustion according to the following equation: C3H6O(l)+4O2(g)3CO2(g)+3H2O(l)+17903kJ a. Use the balance equation, together with the standard heats of formation from the data booklet to calculate the standard heat of formation of acetone (in kJ/mol ). ( 2 marks) b. Calculate the mass of acetone (in grams) required to react with excess oxygen gas to produce 9500 kJ of energy from this combustion reaction. (2 marks) Name the following molecule Question 14 (1 point) Name the following organic molecule Name the following organic molecu Question 16 ( 3 points) There are several structural isomers of the compound 1-chloropentan-1-ol, C5H11ClO Draw line structures for two branched alkyl chain isomers and one straight chain isomer. Give the IUPAC name for one of the branched alkyl chain isomer. (4 marks) Draw the line structures to represent the reactants and products in the formation of butyl propanoate. Give the IUPAC name of the reactants. ( 3 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


