Question
If we are looking at transitions from a much higher state to a much lower state (i.e., n n ), what can we say
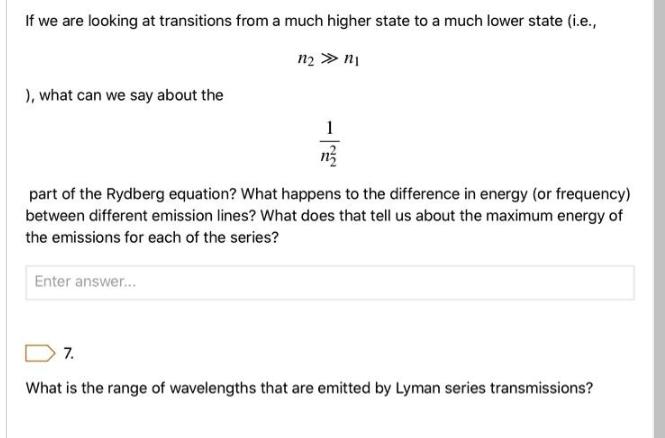
If we are looking at transitions from a much higher state to a much lower state (i.e., n n ), what can we say about the 55 part of the Rydberg equation? What happens to the difference in energy (or frequency) between different emission lines? What does that tell us about the maximum energy of the emissions for each of the series? Enter answer... 7. What is the range of wavelengths that are emitted by Lyman series transmissions?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The range of wavelengths that are emitted by Lyman series transmissions is from 912 nm for n2 to 1216 nm for n2 2 The Lyman series is the first and simplest of the hydrogen spectral series and it corr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



