Question: If we are looking at transitions from a much higher state to a much lower state (i.e., n n ), what can we say
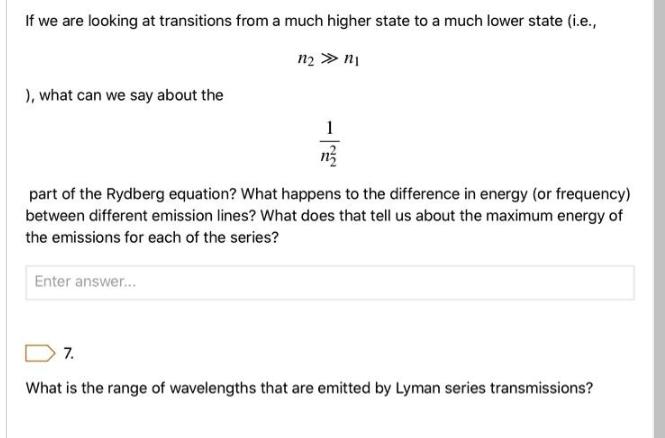
If we are looking at transitions from a much higher state to a much lower state (i.e., n n ), what can we say about the 55 part of the Rydberg equation? What happens to the difference in energy (or frequency) between different emission lines? What does that tell us about the maximum energy of the emissions for each of the series? Enter answer... 7. What is the range of wavelengths that are emitted by Lyman series transmissions?
Step by Step Solution
There are 3 Steps involved in it
The range of wavelengths that are emitted by Lyman series transmissions is from 912 nm for n2 to 1216 nm for n2 2 The Lyman series is the first and simplest of the hydrogen spectral series and it corr... View full answer

Get step-by-step solutions from verified subject matter experts


