Question
Let U, T, S and P denote, respectively, the internal energy, temperature, entropy and pressure of a thermodynamic system. Then a change AF in
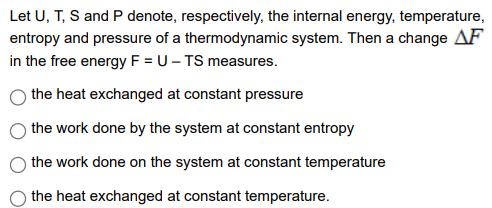
Let U, T, S and P denote, respectively, the internal energy, temperature, entropy and pressure of a thermodynamic system. Then a change AF in the free energy F = U - TS measures. the heat exchanged at constant pressure the work done by the system at constant entropy the work done on the system at constant temperature the heat exchanged at constant temperature.
Step by Step Solution
3.35 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
A First Course In Probability
Authors: Sheldon Ross
9th Edition
978-9332519077, 9332519072
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



