(Multiple choice) (1) The temperature change of two blocks of masses MA and MB is the same...
Question:
(Multiple choice)
(1)
The temperature change of two blocks of masses MA and MB is the same when they absorb equal amounts of heat. It follows that the specific heats are related by
(a) cA = (MA/MB)cB.
(b) cA = (MB/MA)cB.
(c) cA = cB.
(d) None of the above.
(2)
The specific heat of aluminum is more than twice that of copper. Identical masses of copper and aluminum, both at 20°C, are dropped into a calorimeter containing water at 40°C. When thermal equilibrium is reached,
(a) The aluminum is at a higher temperature than the copper.
(b) The aluminum has absorbed less energy than the copper.
(c) The aluminum has absorbed more energy than the copper.
(d) Both (a) and (c) are correct statements.
(3)
In the equation Q = ?U + W (the formal statement of the first law of thermodynamics), the quantities Q and W represent
(a) The heat supplied to the system and the work done by the system.
(b) The heat supplied to the system and the work done on the system.
(c) The heat released by the system and the work done by the system.
(d) The heat released by the system and the work done on the system.
(4)
An ideal gas at one atmosphere pressure and 300 K is confined to half of an insulated container by a thin partition. The partition is then removed and equilibrium is established. At that point, which of the following is correct?
(a) The pressure is half an atmosphere and the temperature is 150 K.
(b) The pressure is one atmosphere and the temperature is 150 K.
(c) The pressure is half an atmosphere and the temperature is 300 K.
(d) None of the above.
(5)
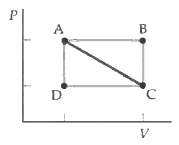
A gas changes its state reversibly from A to C (Figure). The work done by the gas is (a) Greatest for path A ? B ? C.
(b) Least for path A ? C.
(c) Greatest for path A ? D ? C.
(d) The same for all three paths.
(6)
When an ideal gas is subjected to an adiabatic process,
(a) No work is done by the system.
(b) No heat is supplied to the system.
(c) The internal energy remains constant.
(d) The heat supplied to the system equals the work done by the system.
(7)
(a) The heat capacity of a body is the amount of heat it can store at a given temperature.
(b) When a system goes from state 1 to state 2, the amount of heat added to the system is the same for all processes.
(c) When a system goes from state 1 to state 2, the work done on the system is the same for all processes.
(d) When a system goes from state 1 to state 2, the change in the internal energy of the system is the same for all processes.
(e) The internal energy of a given amount of an ideal gas depends only on its absolute temperature.
(f) A quasi-static process is one in which there is no motion.
(g) For any material that expands when heated, Cp is greater than Cv.
(8)
If a system's volume remains constant while undergoing changes in temperature and pressure, then
(a) The internal energy of the system is unchanged.
(b) The system does no work.
(c) The system absorbs no heat.
(d) The change in internal energy equals the heat absorbed by the system.
(9)
When an ideal gas is subjected to an isothermal process,
(a) No work is done by the system.
(b) No heat is supplied to the system.
(c) The heat supplied to the system equals the change in internal energy.
(d) The heat supplied to the system equals the work done by the system.
Step by Step Answer:

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry





