Question: The heat of formation of FeO at 25C (298 K) is -267.3 kJ/mol. The standard entropy of Fe and FeO are 272 and 60.75 W/mol.K.
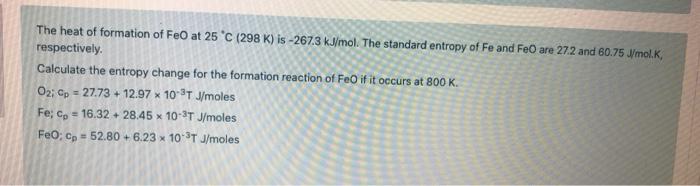
The heat of formation of FeO at 25C (298 K) is -267.3 kJ/mol. The standard entropy of Fe and FeO are 272 and 60.75 W/mol.K. respectively. Calculate the entropy change for the formation reaction of Feo if it occurs at 800 K. O2: op = 27.73 +12.97 * 10-TJ/moles Fe; p = 16.32 -28.45 x 10-3TJ/moles FeO; Cp = 52.80 +6.23 10-3TJ/moles
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


