Benzene can break the ethanol/water azeotrope to produce nearly pure ethanol. Wilson constants for the ethanol (1)/benzene
Question:
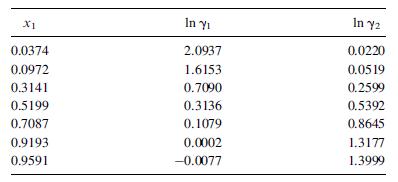
Benzene can break the ethanol/water azeotrope to produce nearly pure ethanol. Wilson constants for the ethanol (1)/benzene (2) system at 45οC are L12 = 0.124 and L21 = 0.523. Use these with theWilson equation to predict liquid-phase activity coefficients over the composition range and compare them, in a plot like Figure 2.12, with the experimental results [Austral. J. Chem., 7, 264 (1954)]:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper
Question Posted:





