It is proposed that oxygen be separated from nitrogen by absorbing and desorbing air in water. Pressures
Question:
It is proposed that oxygen be separated from nitrogen by absorbing and desorbing air in water. Pressures from 101.3 to 10,130 kPa and temperatures between 0 and 100οC are to be used.
(a) Devise a scheme for the separation if the air is 79 mol% N2 and 21 mol% O2.
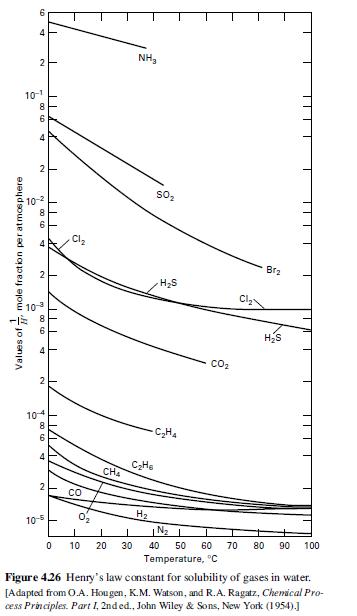
(b) Henry’s law constants for O2 and N2 are given in Figure 4.26. How many batch absorption steps would be necessary to make 90 mol% oxygen? What yield of oxygen (based on the oxygen feed) would be obtained?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper
Question Posted:





