Zirconium, which is used in nuclear reactors, is associated with hafnium, which has a high neutron-absorption cross
Question:
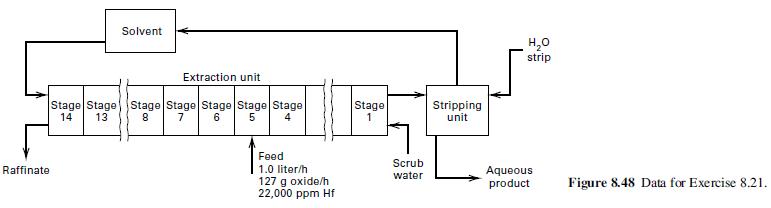
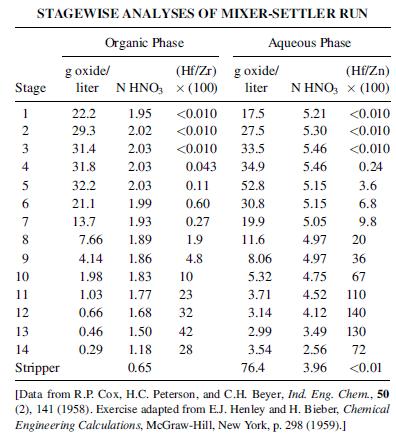
Zirconium, which is used in nuclear reactors, is associated with hafnium, which has a high neutron-absorption cross section and must be removed. Refer to Figure 8.48 for a proposed liquid–liquid extraction process wherein tributyl phosphate (TBP) is used as a solvent for the separation. One L/h of 5.10-N HNO3 containing 127 g of dissolved Hf and Zr oxides per liter is fed to stage 5 of the 14- stage extraction unit. The feed contains 22,000 g Hf per million g of Zr. Fresh TBP enters at stage 14, while scrub water is fed to stage 1. Raffinate is removed at stage 14, while the organic extract phase removed at stage 1 goes to a stripping unit. The stripping operation consists of a single contact between fresh water and the organic phase.
(a) Use the data below to complete a material balance for the process.
(b) Check the data for consistency.
(c) What is the advantage of running the extractor as shown? Would you recommend that all stages be used?
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





