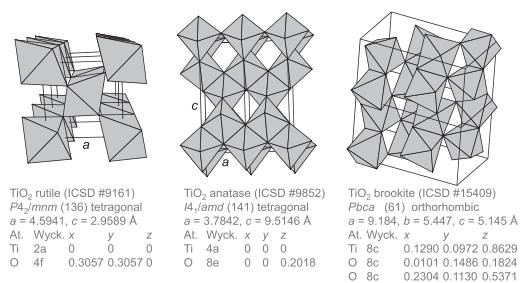
MgF 2 adopts the rutile structure (Figure 1.45) with a = 4.62 and c = 3.04
Question:
MgF2 adopts the rutile structure (Figure 1.45) with a = 4.62 Å and c = 3.04 Å. The bondvalence parameter R0 MgF = 1.581 Å.
(a) Use the bond-valence method to predict the length of the Mg–F bonds.
(b) Use the Born–Mayer equation to estimate the latticeformation energy for MgF2.
(c) Comment on the difference between this value and the value of −2978 kJ/mol obtained from a Born–Haber cycle. Is the agreement between calculated and experimental values similar to that observed for the alkali-metal halides?
(d) Would you expect the lattice-formation energy of rutile (TiO2) to be lower (more stable) than MgF2?
Figure 1.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:





