Consider the pK a values of the following constitutional isomers: Using the rules that we developed in
Question:
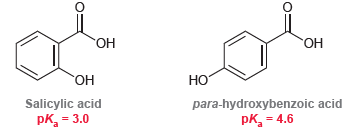
Using the rules that we developed in this chapter (ARIO), we might have expected these two compounds to have the same pKa. Nevertheless, they are different. Salicylic acid is apparently more acidic than its constitutional isomer. Can you offer an explanation for this observation?
Transcribed Image Text:
но. Но. но Он НО Salicylic acid pk = 3.0 para-hydroxybenzoic acid pK = 4.6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
When salicylic acid is d...View the full answer

Answered By

Lilian Nyambura
Hi, am Lilian Nyambura, With extensive experience in the writing industry, I am the best fit for your writing projects. I am currently pursuing a B.A. in Business Administration. With over 5 years of experience, I can comfortably say I am good in article writing, editing and proofreading, academic writing, resumes and cover letters. I have good command over English grammar, English Basic Skills, English Spelling, English Vocabulary, U.S. English Sentence Structure, U.K. or U.S. English Punctuation and other grammar related topics. Let me help you with all your essays, assignments, projects, dissertations, online exams and other related tasks. Quality is my goal.
4.80+
378+ Reviews
750+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Offer an explanation for the observation that 4-chloropyridine is more reactive toward nucleophiles than 3-chloropyridine.
-
Offer an explanation for the observation that 4-chloropyridine is more reactive toward nucleophiles than 3-chloropyridine.
-
Is it always possible to reduce a combination of capacitors to one equivalent capacitor with the rules developed in this chapter? Explain.
-
In using the bolt cutter shown, a worker applies two 300-N forces to the handles. Determine the magnitude of the forces exerted by the cutter on the bolt. 300 N 12 mm 24 mm E 24 mm 460 mm 96 mm 300 N...
-
What services are provided by investment banks? Do they compete with commercial banks?
-
Determine the steady-state response for the input \(x(n)=\sin (\omega n) u(n)\) of the filters described by (a) \(y(n)=x(n-2)+x(n-1)+x(n)\) (b) \(y(n)-\frac{1}{2} y(n-1)=x(n)\) (c) \(y(n)=x(n-2)+2...
-
Audience Scores on Rotten Tomatoes The variable AudienceScore in the dataset HollywoodMovies gives audience scores (on a scale from 1 to 100) from the Rotten Tomatoes website. The five number summary...
-
Name and briefly describe the five product mix pricing decisions.
-
A 250 MW coal-fired power plant has a heat rate of 9,300 BTUs. What is its 1st Law efficiency?
-
Pin B has a mass m and slides along the slot in the rotating arm OC and along the slot DE which is cut in a fixed horizontal plate. Neglecting friction and knowing that rod OC rotates at the constant...
-
Identify reagents that can be used to achieve each of the following transformations: (a) To convert 1-hexene into a primary alcohol (b) To convert 3, 3-dimethyl-1-hexene into a secondary alcohol (c)...
-
Consider the following compound with molecular formula C 4 H 8 O: (a) Draw a constitutional isomer that you expect will be approximately one trillion (10 12 ) times more acidic than the compound...
-
In working for a local retail store, you have developed the following estimated regression equation: where: y = Weekly sales x1 = Local unemployment rate x2 = Weekly average high temperature x3 =...
-
3. Simplify the logarithmic expression: 2 - 3 log4 p log4 (d w) + hlogy - [4 marks] 4. The fifth term of an arithmetic progression is the same value as the third term of a geometric progression that...
-
Moshe's Social Security benefit at full retirement age will be $1,576.00 per month. Determine what his monthly benefit will be if he begins collecting 21 months early.
-
7. A rectangular box measures 18 in by 24 in by 30 in, as shown. What is the longest stick that can be placed inside the box? Round to the nearest inch. 18 in 24 in 30 in
-
4. a) What is the value of cos 30 ? What is the value of sin 60 ? b) Explain why cos 30 and sin 60 are related in this way. (You may want to draw a triangle with these angles to help you.) c) Using...
-
A community athletic club holds an election to select a president and vice president. The nominations for selection include 4 females and 3 males. What is the probability that a female is elected...
-
Determine the range and standard deviation of the set of data. When appropriate, round standard deviations to the nearest hundredth. 4, 8, 9, 11, 13, 15
-
If your school has a subscription to the FASB Codification, go to aaahq.org/ ascLogin.cfm to log in and prepare responses to the following. (a) What is the stock dividend? (b) What is a stock split?...
-
We saw in Section 17.4 that ketones react with NaBH 4 to yield alcohols. We?ll also see in Section 22.3 that ketones react with Br 2 to yield a-bromo ketones. Perhaps surprisingly, treatment with...
-
Give IUPAC name for the following compounds: (c) , (b) (a) CHCH,CHCHCH CH CH2CH2CH3 -CH CH C (e) . (f) (d) Br Br
-
Draw structures corresponding to the following IUPAC name: (a) (Z)-2-Ethyl-2-buten-1-o1 (b) 3-Cyclohexen-1-o1 (c) trans-3-Chlorocycloheptanol (d) 1, 4-Pentanediol (e) 2, 6-Dimethylphenol (f)...
-
Show that for the linear regression model Y = XTB + , the leave-one-out cross validation identity: where H = n (Yi - (-i)) n Yi - i=1 i 2 Hii 2 " X(XX)-XT is the hat matrix and H; is the ith diagonal...
-
DQ: Chapter 3 talks about GDP. If California was a separate country, it would rank as the 5th largest economy after the US, Japan, China, and Germany. Explain what GDP is and how is it different than...
-
then A-1 = Given b= A -13-11 -5 14 -47 1 -3 10 solve Axb using A-.

Study smarter with the SolutionInn App


