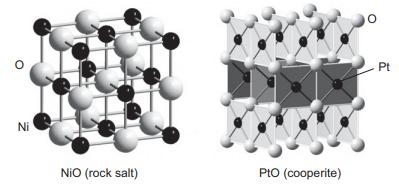
NiO adopts the cubic NaCl-type structure while PtO adopts the cooperite structure shown below. (a) What factor
Question:
NiO adopts the cubic NaCl-type structure while PtO adopts the cooperite structure shown below.
(a) What factor do you think is responsible for the differing crystal chemistry preferences of these two compounds? Consider the splitting and occupation of the d orbitals for each compound.
(b) Which structure type do you think PdO will adopt? Would it be possible to tell from a magnetic measurement?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:





