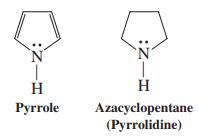
Pyrrole is a much weaker base than azacyclopentane (pyrrolidine) for which of the following reasons? (a) The
Question:
Pyrrole is a much weaker base than azacyclopentane (pyrrolidine) for which of the following reasons?
(a) The nitrogen in pyrrole is more electropositive than that in pyrrolidine;
(b) Pyrrole is a Lewis acid;
(c) Pyrrole has four electrons;
(d) Pyrrolidine can give up the proton on the nitrogen atom more readily than can pyrrole;
(e) Pyrrole is aromatic.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





