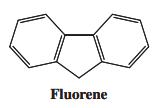
The hydrocarbon with the common name fluorene is acidic enough (pK a 23) to be a
Question:
The hydrocarbon with the common name fluorene is acidic enough (pKa ≈ 23) to be a useful indicator in deprotonation reactions of compounds of greater acidity. Indicate the most acidic hydrogen(s) in fl uorene. Draw resonance structures to explain the relative stability of its conjugate base.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:




