When methyl ketones are treated with a halogen in the presence of base, the three hydrogen atoms
Question:
When methyl ketones are treated with a halogen in the presence of base, the three hydrogen atoms on the methyl carbon are replaced to give a CX3-substituted ketone. This product is not stable under the basic conditions and proceeds to react with hydroxide, ultimately furnishing the carboxylic acid (as its conjugate base) and a molecule of HCX3, which has the common name haloform (i.e., chloroform, bromoform, and iodoform, for X 5 Cl, Br, and I, respectively). For example:
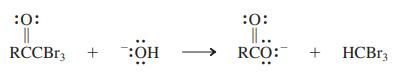
Propose a series of mechanistic steps to convert the tribromoketone into the carboxylate. What is the leaving group? Why do you think this species is capable of acting as a leaving group in this process?
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore




