At a particular temperature, 8.1 moles of NO 2 gas are placed in a 3.0-L container. Over
Question:
At a particular temperature, 8.1 moles of NO2 gas are placed in a 3.0-L container. Over time the NO2 decomposes to NO and O2:
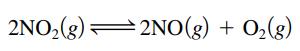
At equilibrium the concentration of NO(g) was found to be 1.4 mol/L. Calculate the value of K for this reaction.
Transcribed Image Text:
2NO₂(g) 2NO(g) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
The value of K ra...View the full answer

Answered By

Willis Omondi
Hi, I'm Willis Omondi, a proficient and professional academic writer. I have been providing high-quality content that best suits my clients and completing their work within the deadline. All my work has been 100% plagiarism-free, according to research from my services, especially in arts subjects and many others
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Two moles of an ideal gas are placed in a container whose volume is 8.5 10-3 m3. The absolute pressure of the gas is 4.5 105 Pa. What is the average translational kinetic energy of a molecule of...
-
At a particular temperature and pressure, a helium gas contains 5 10 25 atoms/m 3 . If a 10-kV/m field applied to the gas causes an average electron cloud shift of 10 8 m, find the dielectric...
-
At a particular temperature the value of [Ba 2+ ] in a saturated solution of barium sulfate is 1.035x10 -5 M . Starting with this information, calculate the K sp of barium sulfate at this temperature.
-
State whether the following statement are True or False An increase in the owner(s) equity, in the absence of any further investment by the owners is typically effected by sale transactions.
-
Explain the functions of distribution channels.
-
If A, B, and C are mutually exclusive events, is it possible for P (A) = 0.3, P (B) = 0.4, and P (C) = 0.5? Why or why not?
-
Show that by writing the velocity in terms of the similarity variable \(\eta\) and the function \(f(\eta)\), the momentum equation for boundary layer flow on a flat plate (Eq. 9.9b) can be written as...
-
Indicate whether each of the following actions relates to (a) Managing liquidity and cash flows, (b) Recognition of liabilities, (c) Valuation of liabilities, (d) Classification of liabilities, or...
-
1. For the object shown in figure 1,(a) Find the moment of inertia about the x axis.(b) Find the moment of inertia about the y axis.(c) Find the product of inertia.(d) Find the polar moment of...
-
In which quarter(s) was the percentage change in velocity positive? Choose one or more: A. Q1 2020 B. Q2 2020 C. Q3 2020 Part 2 (2 points) Lets focus on the second quarter since the change in...
-
A sample of solid ammonium chloride was placed in an evacuated chamber and then heated, causing it to decompose according to the following reaction: In a particular experiment, the equilibrium...
-
Methanol, a common laboratory solvent, poses a threat of blindness or death if consumed in sufficient amounts. Once in the body, the substance is oxidized to produce formaldehyde (embalming fluid)...
-
Name each species. 1. P3 2. Sr2+
-
In forecasting human resources demand, what quantitative or qualitative techniques would be most appropriate for the following: 1. Small versus large companies 2. Industries undergoing rapid change...
-
A student creates a two-point source interference pattern in a ripple tank with two sources operating in phase. A point on the eighth nodal line is 1.25 m from the centre of the two sources and 48.0...
-
What role would Southwest development play in overcoming resistance to change?
-
What is the difference between HRM and IHRM? 2. To what extent should multinationals adapt their HRM practices in foreign locations to achieve cultural fit? What should be considered before deciding?...
-
A third harmonic forms in a pipe (from a sound wave of frequency 501 Hz) which is open at one end and closed at the other end. Take the speed of sound as v=345 m s. How long is the pipe in metres?
-
A gas occupying a volume of 725 mL at a pressure of 0.970 atm is allowed to expand at constant temperature until its pressure reaches 0.541 atm. What is its final volume?
-
You are the newly appointed tax practitioner to complete Emilys tax return and have downloaded the prefill report for Emilys tax return (hint, you can read what a prefill report is here (Links to an...
-
For each of the following compounds, predict the energy barrier to rotation (looking down any one of the C-C bonds). Draw a Newman projection and then compare the staggered and eclipsed...
-
In each case below, identify the highest and lowest energy conformations. In cases where two or three conformations are degenerate, draw only one as your answer. (a) (b) (c) (d)
-
Compare the three staggered conformations of ethylene glycol. The anti conformation of ethylene glycol is not the lowest energy conformation. The other two, staggered conformations are actually lower...
-
You anticipate the receipt of money in 200 days, which you will use to purchase stocks in a particular company. The stock is currently selling for $51 and will pay a $0.5 dividend in 50 days and...
-
1) Based on the stock chart for Michaels Companies Inc, what do you think the short and long-term growth potentials are for this company? (discuss the advantages/disadvantages) Link to the stock...
-
After being drafted in the first round of the NFL draft, a star defensive end invests his signing bonus of $9,827,000.00 in a mutual fund. The fund pays on average 7.00% APR. The player will not...

Study smarter with the SolutionInn App


