The vapor pressures of several solutions of waterpropanol (CH 3 CH 2 CH 2 OH) were determined
Question:
The vapor pressures of several solutions of water–propanol (CH3CH2CH2OH) were determined at various compositions, with the following data collected at 45οC:
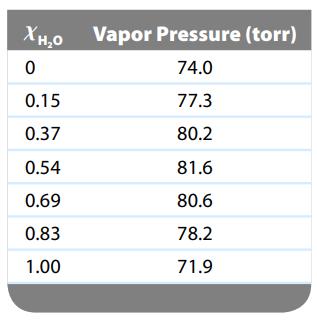
a. Are solutions of water and propanol ideal? Explain.
b. Predict the sign of ΔHsoln for water–propanol solutions.
c. Are the interactive forces between propanol and water molecules weaker than, stronger than, or equal to the interactive forces between the pure substances? Explain.
d. Which of the solutions in the data would have the lowest normal boiling point?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:





