What mass of phosphorus is present in 5.00 moles of each of the compounds in Exercise 54?
Question:
What mass of phosphorus is present in 5.00 moles of each of the compounds in Exercise 54?
Data in Exercise 54?
Calculate the molar mass of the following substances.
a. 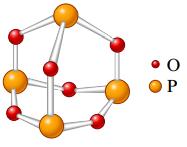
b. Ca3(PO4)2
c. Na2HPO4
Transcribed Image Text:
0 эр
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
Molecular compound PO 1 mole PO 36460 gmol x 500 moles 17...View the full answer

Answered By

Enock Oduor
I am a chemist by profession, i coach high school students with their homework, i also do more research during my free time, i attend educational and science fair seminars where i meet students and do some projects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
What mass of phosphorus is needed to dope l.0 g of silicon to the extent described in Sample Problem 41-6?
-
Phosphorus is present in seawater to the extent of 0.07 ppm by mass. If the phosphorus is present as phosphate, PO43-calculate the corresponding molar concentration of phosphate in seawater.
-
What are the moles of substances present at equilibrium at 450oC if 1.00 mol N2 and 4.00 mol H2 in a 10.0-L vessel react according to the following equation? The equilibrium constant Kc is 0.153 at...
-
If the working papers correlating with this textbook are not used, omit Problem 2-5B. The following records of A-Aall Electronic Repair are presented in the working papers: Journal containing...
-
Choose an appropriate forecasting technique for the data in the Excel file Federal Funds Rates and find the best forecasting model. Explain how you would use the model to forecast and how far into...
-
The top surface of a compartment consists of very smooth (A) and highly roughened (B) portions, and the surface is placed in an atmospheric airstream. In the interest of minimizing total convection...
-
A fan (see Fig. P5.86) has a bladed rotor of 12 -in. outside diameter and 5-in. inside diameter and runs at \(1725 \mathrm{rpm}\). The width of each rotor blade is \(1 \mathrm{in}\). from blade inlet...
-
Virginia Partners, Ltd. (Virginia Partners), a limited partnership organized under the laws of Florida, conducted business in Kentucky but failed to register as a foreign limited partnership, as...
-
What are the potential benefits of integrating elements from both positive psychology and clinical psychology? Please include references
-
To raise operating funds, North American Courier Corporation sold its building on January 1, 2011, to an insurance company for $500,000 and immediately leased the building back. The lease is for a...
-
Using the general solubility rules given in Table 4.1, name three reagents that would form precipitates with each of the following ions in aqueous solution. Write the net ionic equation for each of...
-
What mass of compound is present in 5.00 moles of each of the compounds in Exercise 54? Data In Exercise 54? Calculate the molar mass of the following substances. a. b. Ca 3 (PO 4 ) 2 c. Na 2 HPO 4 0
-
In what way are an individuals pants with four pockets similar to a parent company with three subsidiaries? Explain, with reference to intercompany revenues and expenses.
-
Sprout Inc.'s direct materials cost is $492,400, direct labor cost is $397,800, indirect materials cost is $2,250, indirect labor cost is $4,750, and other factory overhead costs are $79,300. What is...
-
How is fixed cost treated in marginal costing? Discuss the concept of direct and indirect costs in marginal costing.
-
Item2 Item 2 When a CPA firm examines projected financial statements, the firm's report should include a separate paragraph that: Multiple Choice Limits distribution of the projections to specified...
-
Percy's Porcelain Works manufactures fine vases. The company produces two designs: a square vase and a round vase. The planning team is discussing the coming year's activities. To start the...
-
How can work-life balance, job experience and loyalty impact the culture of your organization, and the impact of such differences on succession planning. Elaborate.
-
Crystalline silicon has a cubic structure. The unit cell edge length is 543 pm. The density of the solid is 2.33 g/cm3. Calculate the number of Si atoms in one unit cell?
-
A liquid flows upward through a valve situated in a vertical pipe. Calculate the differential pressure (kPa) between points A and B. The mean velocity of the flow is 4.1 m/s. The specific gravity of...
-
Show the products of thesereactions: CH3 HBr a) CH,CHCH-CH2 HBr b) PhC CH 2 HBr
-
Show the products of thesereactions: H,SO,, H,SO4 b) + H,0 + H,0 H,SO. + HO
-
Show all of these steps in the mechanism for the addition of water to propene catalyzed by sulfuric acid. Explain whether propene or phenylethene (PhCH = CH2) has a faster rate in this reaction:
-
Figure out, salaries, net income, add net income with retained earnings and totally stockholder equity , ?accounts payable and total liabilities and stockholders equity RILEY, INCORPORATED Statement...
-
Determine the material inventory balance at the end of may? Received Issued Receiving Received Materials Report Number Received Quantity Unit Price Requisition Number Issued Quantity Issued Balance...
-
During October 2 0 2 3 , Fern Field Farms, Inc. received $ 1 0 , 0 0 0 from customers in exchange for fruit and vegetables. During the same month, the company paid $ 2 , 0 0 0 to employees, $ 5 0 0...

Study smarter with the SolutionInn App


